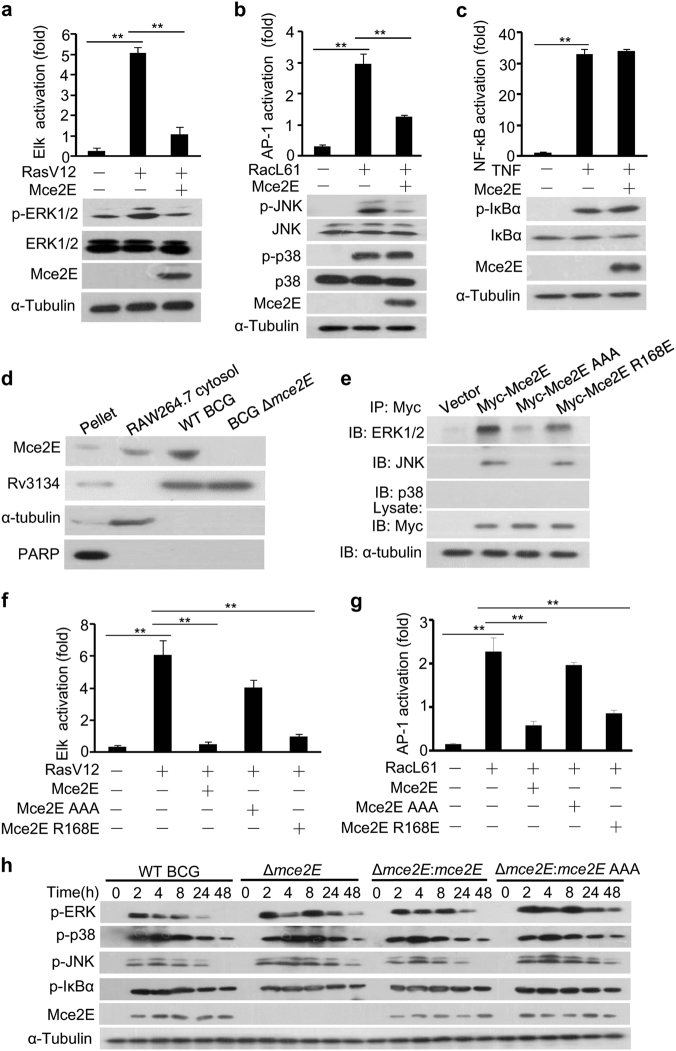
Fig. 1.
Mtb Mce2E suppresses the ERK and JNK signaling pathways in a D motif-dependent manner. a–c Luciferase reporter assay (top) and immunoblot analysis (bottom) of Elk (a), AP-1 (b), or NF-κB (c) activation by Mtb Mce2E in HEK293T cells transfected with the empty vector or vector encoding Myc-tagged Mce2E. The ERK pathway was activated by co-expression of constitutively active RasV12. The JNK and p38 MAPK pathways were activated by co-expression of constitutively active RacL61. The NF-κB pathway was stimulated by TNF treatment. d RAW264.7 cells were infected with wild-type (WT) BCG or BCG Δmce2E at a multiplicity of infection (MOI) of 10 for 4 h. Cells were collected to obtain the cytosolic fraction, pellet containing the RAW264.7 nuclei and BCG, and bacteria for immunoblot analysis of Mce2E expression. e Immunoblot analysis of proteins immunoprecipitated with anti-Myc antibodies from lysates of HEK293T cells transfected with the empty vector or vectors encoding Myc-tagged Mce2E or its mutants. f Luciferase reporter assay of Elk activation by Mce2E or its mutants in HEK293T cells. The ERK pathway was stimulated as in c. g Luciferase reporter assay of AP-1 activation by Mce2E or its mutants in HEK293T cells. The JNK and p38 pathways were stimulated as in d. h Immunoblot analysis of phosphorylated ERK, JNK, p38, IκBα, Mce2E and total α-tubulin (loading control throughout) in RAW264.7 cells. Cells were infected with wild-type BCG (WT BCG) or BCG Δmce2E (Δmce2E) or BCG Δmce2E complemented with WT mce2E (Δmce2E:mce2E) or mce2E AAA (Δmce2E:mce2E AAA) at a MOI of 10 for 0–48 h. *P < 0.05 and **P < 0.01 (two-tailed unpaired t test). Data are representative of experiments with at least three independent biological replicates (mean and s.e.m. of n = 3 cultures)