Fig. 5.
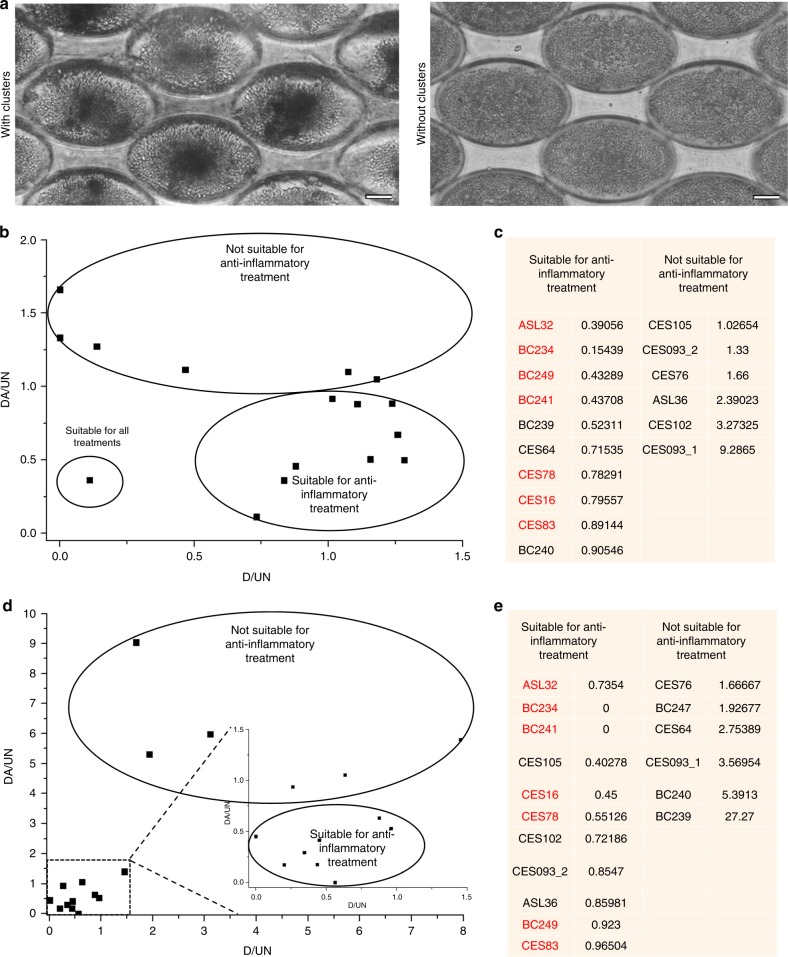
Evaluation of Combinatorial DA treatment on patient-derived CTC models using the Cluster assay. a Representative images of microwell array with clusters (left) and without clusters (right). Scale bar is 50 µm. b Scatter plot demonstrating the killing efficacy in clinical cohorts. DA/UN = viability of putative CTCs in patient-derived models after 72 h of treatment under combinatorial DA treatment, relative to untreated samples. D/UN = viability of putative CTCs in patient-derived models after 72 h of treatment under treatment with doxorubicin alone, relative to untreated samples. The response in terms of killing efficacy is heterogeneous with samples of comparable response grouped in black. c List of patients suitable or unsuitable for anti-inflammatory combinatorial DA treatment based on viability ratio. Patient samples that responded in terms of both killing efficacy and CSC reduction are marked in red. d Scatter plot demonstrating the reduction of CSCs in clinical cohorts. DA/UN = CSC counts of putative CTCs in patient-derived models after 72 h of treatment under combinatorial DA treatment, relative to untreated samples. D/UN = CSC counts of putative CTCs in patient-derived models after 72 h of treatment under treatment with doxorubicin alone, relative to untreated samples. The response in terms of reduction of CSCs is heterogeneous with samples of comparable response grouped in black. e List of patients suitable or unsuitable for anti-inflammatory combinatorial DA treatment based on CSC counts. Patient samples that responded in terms of both killing efficacy and CSC reduction are marked in red