Fig. 1.
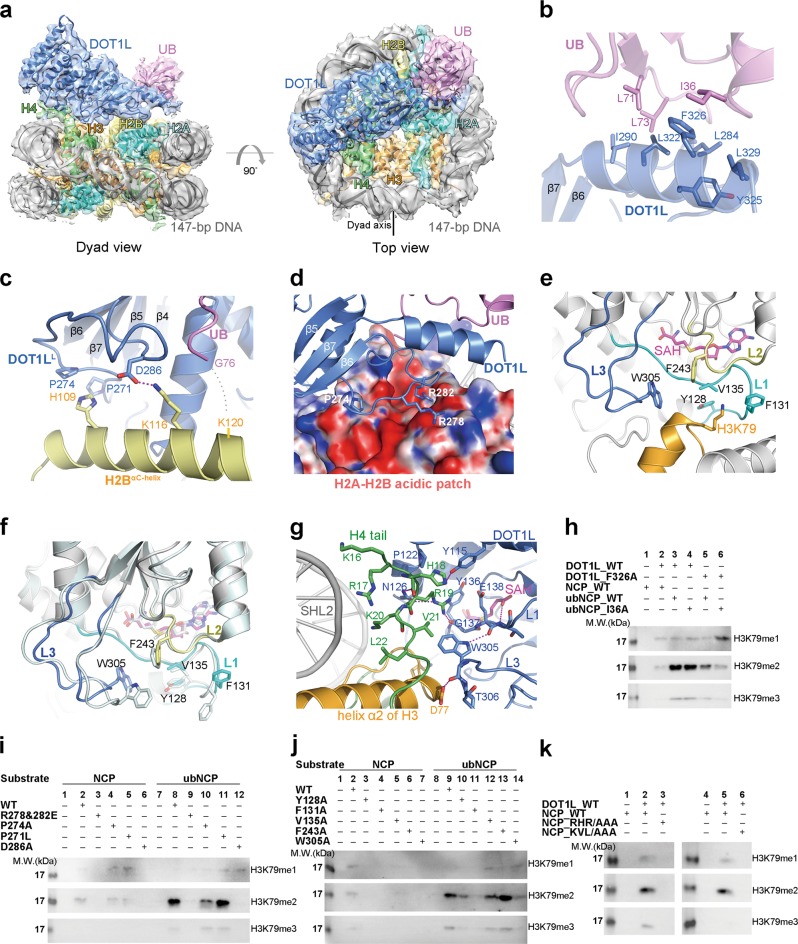
Structural characterization of the recognition of the H2BK120ub1 nucleosome by DOT1L. a Overall structure of the DOT1L-ubNCP complex shown from two orthogonal views. The cryo-EM map was segmented and colored according to the respective components of the DOT1L-ubNCP complex. b Detailed view of the DOT1L-ubiquitin interface, the related EM density map is shown in Supplementary information, Fig. S8b. c The recognition interface between DOT1LL and the ubiquitin-conjugated C-terminal helix of histone H2B, the related EM density map is shown in Supplementary information, Fig. S8c. d DOT1L Arg278 and Arg282 interact with the acidic patch of histone H2A-H2B (shown with electrostatic surface potential), the related EM density map is shown in Supplementary information, Fig. S8e. e Detailed view of the interface between the active site of DOT1L and the nucleosomal H3K79, the related EM density map is shown in Supplementary information, Fig. S9a-e. f Comparison of the crystal structure of DOT1L alone (PDB code: 1NW3, colored in pale cyan) and the cryo-EM structure of DOT1L in complex with ubNCP (colored as in e) reveals conformational changes of the active site of DOT1L upon binding to nucleosome. g Detailed interactions between the histone H4 tail and the active site of DOT1L. The related EM density map is shown in Supplementary information, Fig. S9f. The hydrogen-bonds are shown as magenta dash lines. h Mutational studies on the function of the interacting Phe326DOT1L-Ile36Ub residues in the H3K79 methylation of ubNCP through the HMT assay. Input of the HMT reactions is shown in Supplementary information, Fig. S8f. i HMT assays carried out with DOT1L mutants that disrupt the interactions between DOT1L and the histone H2A-H2B dimer. Input of the HMT reactions is shown in Supplementary information, Fig. S8h. j Mutations of the DOT1L residues that make direct contact with the adjacent region of H3K79 abrogate the HMT activity of DOT1L to varying degrees. Input of the HMT reactions is shown in Supplementary information, Fig. S9g. k DOT1L fails to methylate the mutant nucleosomes that have alanine substitutions of the DOT1L-interacting H4 residues. Input of the HMT reactions is shown in Supplementary information, Fig. S9h