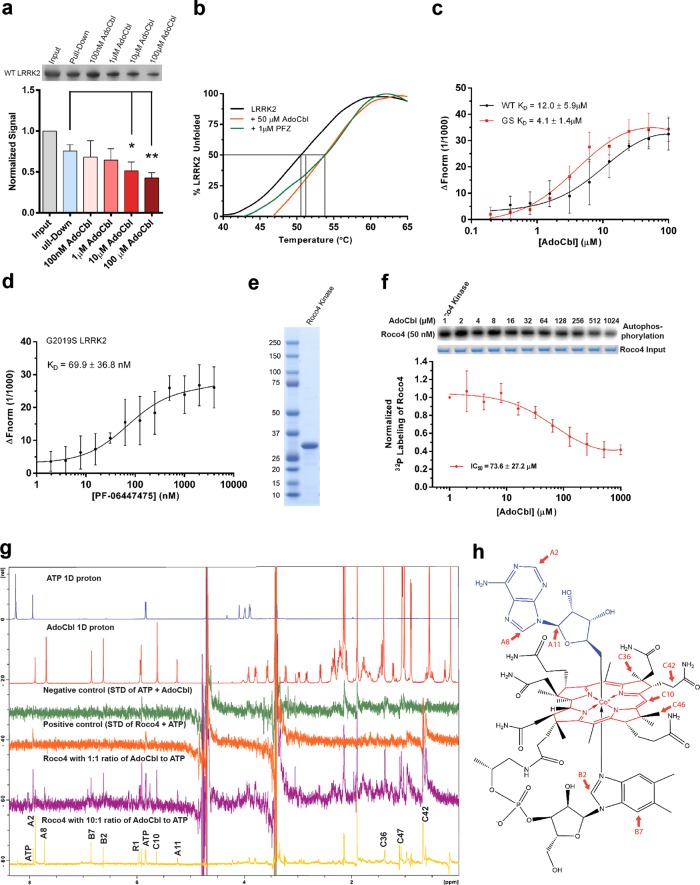
Fig. 2.
Direct binding of AdoCbl to LRRK2 protein. a Binding of strep-tagged LRRK2 to AdoCbl-agarose in the presence of AdoCbl. Input represents the amount of protein that was added to beads, while pull-down denotes the amount of protein left on the beads after washes. Significance was calculated by one-way ANOVA using the mean (±s.d.) of three biological replicates. *p ≤ 0.05, **p ≤ 0.005. b Thermal shift assays showing melting temperatures of strep-LRRK2 in the presence of AdoCbl or PF-06447475. c Microscale thermophoretic analysis of the interaction between AdoCbl or d PF-06447475 with strep-tagged LRRK2. e Coomassie stained SDS-PAGE of the Roco4 kinase domain purified from E. coli. f Dose-response curve of Roco4 kinase activity as a function of AdoCbl. g ATP STD-NMR shows direct binding of AdoCbl to the Roco4 kinase domain and competition with ATP. From top to bottom, the spectra are as follows: 1D 1 H NMR of ATP (blue), AdoCbl (red), STD negative control with ATP + AdoCbl only (green), STD positive control with ATP and Roco4 kinase domain (orange), STD of AdoCbl and Roco4 kinase domain with 1:1 ratio of AdoCbl to ATP (purple), and STD of AdoCbl and Roco4 kinase domain with 10:1 ratio of AdoCbl to ATP (yellow). AdoCbl protons showing strong STD signals are labeled with assignment. All experiments were collected at 4 °C on a Bruker 800 MHz spectrometer equipped with a cryoprobe. h Protons with strong STD signals (highlighted in red) mapped onto the structure of AdoCbl. The NMR assignment and nomenclature of vitamin B12 is from Summers et al.94 Data points in a, c, d, f represent the mean (±s.d.) of three biological replicates