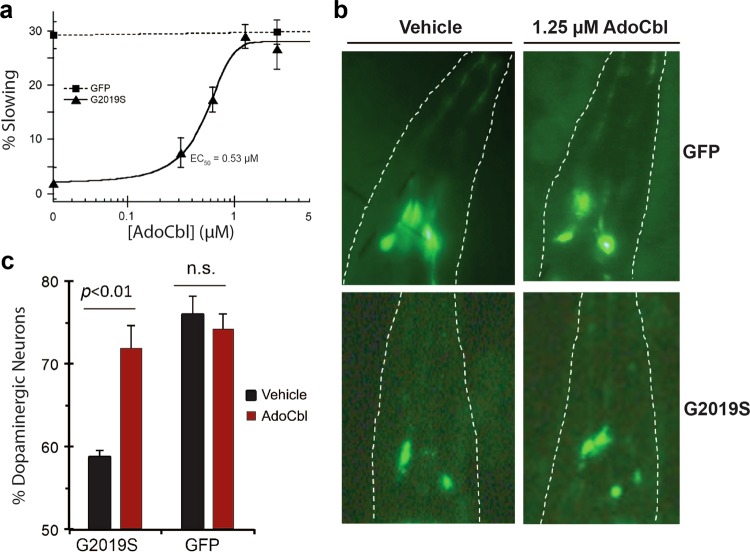
Fig. 5.
AdoCbl rescues mutant human LRRK2-induced behavioral defects and dopaminergic neurodegeneration in C. elegans. a AdoCbl dose dependently rescues the loss of basal slowing response in transgenic hLRRK2-G2019S C. elegans. Age-synchronized nematodes expressing GFP marker only or additionally hLRRK2-G2019S in dopaminergic neurons were treated with either vehicle or AdoCbl in liquid culture during the larval stage L1 to L4 (3 days), followed by growth on NGM plates for 3 days prior to behavior assay. Basal slowing response was assayed on NGM plates using an unbiased machine-vision analysis system (WormLab) as the percent slowing in body bends per 20 s in the presence vs. the absence of bacterial lawn. Data represent the mean (±s.d.) of three biological replicates, each with 20–25 worms per treatment condition. b AdoCbl treatment attenuated the loss of dopaminergic neurons induced by hLRRK2-G2019S in C. elegans. Representative fluorescence images of dopaminergic neurons (CEP neurons within the outlined head region) in transgenic C. elegans expressing GFP marker only or additionally hLRRK2-G2019S following treatment with either vehicle or 1.25 µM AdoCbl. Age-synchronized nematodes were treated with either vehicle or AdoCbl in liquid culture during the larval stage L1 to L4 (3 days), followed by growth on NGM plates for 9 days. GFP-tagged dopaminergic neurons in live animals were counted under a fluorescence microscope. c Quantification of percent dopaminergic neurons survived. Data are presented as the mean (±s.d.) of three biological replicates, each with approximately 30–50 worms per treatment condition. P < 0.01, Student’s t-test. n.s., not statistically significant