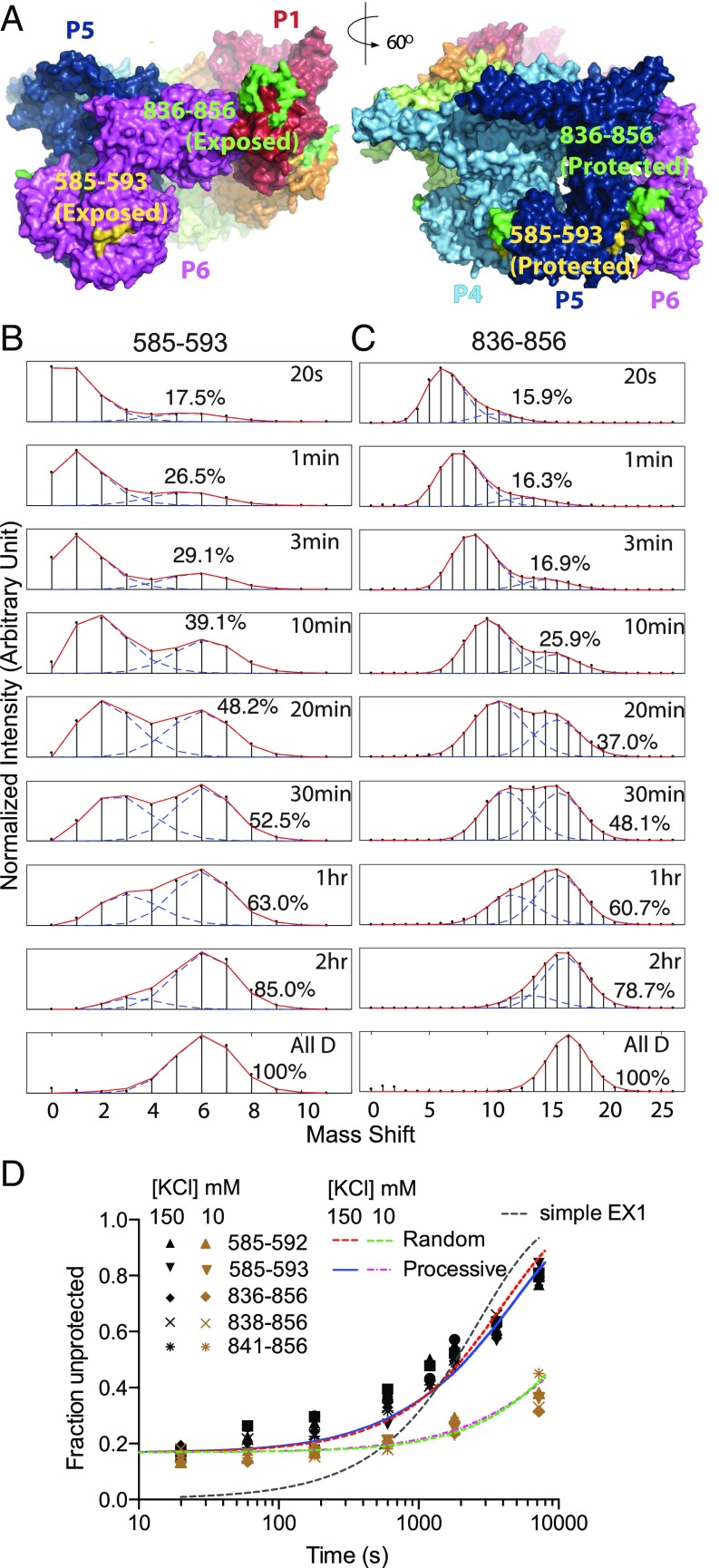
Fig. 4.
Heterogeneous HX of noncanonical interface peptides. (A) The offset noncanonical P1–P6 interface (Left) and a canonical P5–P6 interface for comparison, noting the peptides measured in B and C. (B and C) In the AMPPNP state, segments that are symmetrically paired in the five canonical interfaces but offset and solvent exposed in the noncanonical interface exhibit bimodal HX MS envelopes due to their different HX rates. (D) The less protected (unpaired) population fraction for these and other overlapping peptides plotted versus H-to-D labeling time. The bimodality is not well fit by a simple EX1 dynamic exposure HX mechanism (dashed black curve). A 17% less protected population is already present at early HX time (20 s to 2 min), equal to the 1:5 noncanonical to canonical interface ratio indicated by cryo-EM. To focus selectively on the HX of the interface peptides and avoid the later dynamic protomer interchange, the experimental pD was increased to 8.9 (25 °C, AMPPNP-bound state) to speed HX by a factor of 10. The slow later upsweep shows that protomers interchange and time share the noncanonical position, and can be well fit by either processive or random reassociation models (SI Appendix, Fig. S5), but on a very long time scale (∼1 h) that is nonfunctional. A test at even lower salt where the hexamer is more stable makes protomer interchange even slower (lower curves with brown data points; 0.02 min−1 at 10 mM KCl and 0.09 min−1 at 150 mM KCl), supporting the dissociation interpretation.