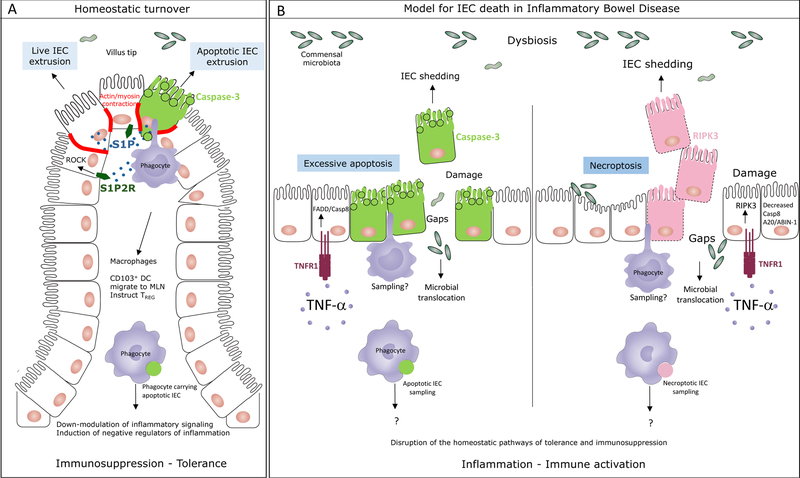
Figure 1. The mode of cell death in the intestinal epithelium impacts intestinal homeostasis.
A. Under homeostatic conditions, the intestinal epithelium undergoes natural turnover by continuous replacement of intestinal epithelial cells (IEC). Experimental evidence supports both apoptotic and non-apoptotic IEC extrusion at locations of overcrowding at the villi tips of the small intestine or luminal face of the crypts in the colon. Both of these processes require sphingosine 1-phosphate (S1P) signaling through S1P2 receptor (S1P2R) and resultant Rho associated kinase (ROCK) dependent actin/myosin contraction. ROCK can be cleaved and activated by caspase-3 during apoptosis to phosphorylate myosin light chain and induce apoptotic membrane blebbing. MHC class II+ CD11c+ intestinal lamina propria phagocytes, either macrophages or CD103+CD11b– dendritic cells (DC) sample the apoptotic IEC prior to their exclusion and respond by executing a broad program of immunosuppression. Apoptotic IEC carrying CD103+ DC also express negative regulators of inflammation such as the IBD susceptibility gene TNFAIP3 encoding A20. These cells express the lymph node homing receptor CCR7 and induce the emergence of regulatory CD4 T (TREG) cells. B. A proposed model for the types of IEC death that might occur during in inflammatory bowel disease. Increased levels of TNF-α in the intestinal lamina propria of patients with IBD induces IEC shedding that can be a result of excessive and unregulated apoptosis mediated by FADD and caspase-8 downstream of TNF receptor (TNFR1) engagement on IEC. Based on studies in mouse models, under conditions when caspase-8 or A20/ABIN-1 activity is impaired, TNF-α signaling through TNFR1 induces necroptosis mediated by the receptor interacting kinase 3 (RIPK3). Dying IEC are likely samples by intestinal phagocytes although formal proof of this process and its consequences is yet to come. In the absence of homeostatic apoptosis, it is expected that the immunosuppression and tolerance program in phagocytes, imparted by apoptotic IEC sampling, would be disrupted. Necroptosis is inflammatory in nature and would be expected to lead to the activation of intestinal phagocytes. Excessive apoptosis and necroptosis would compromise barrier integrity and lead to the translocation of the commensal microbiota into the sterile lamina propria leading to more inflammation.