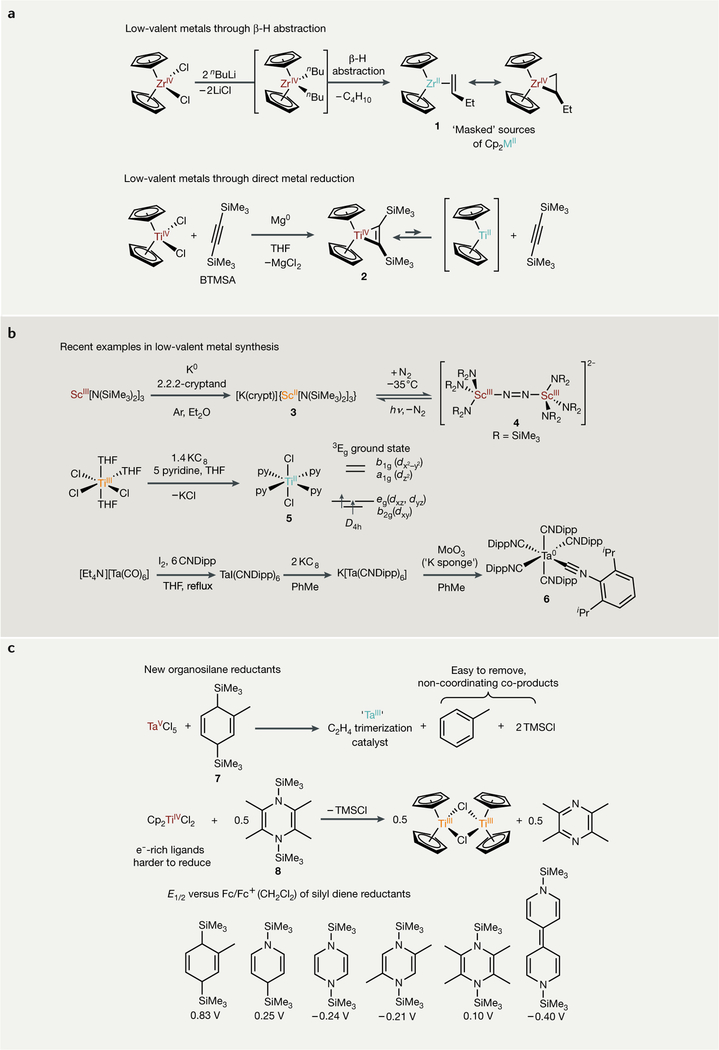
Fig. 2 |. Examples of synthetic routes to low-valent early transition metal complexes.
a | Classical reduction routes involving strong reductants. b | Representative examples of modern low-valent metal synthesis. c | New organosilane reductants for low-valent metal synthesis and catalysis. These routes demonstrate the diverse array of reduction methods available and the common ability of π-accepting ligands to stabilize low-valent early transition metal complexes. Red-coloured metals are in their highest oxidation state, orange-coloured metals are one-electron reduced, and teal-coloured metals are two-electron reduced. hv, irradiation; Dipp, 2,6-diisopropylphenyl; E1/2, half-wave potential; Fc, ferrocene; THF, tetrahydrofuran; TMSCl, trimethylsilyl chloride.