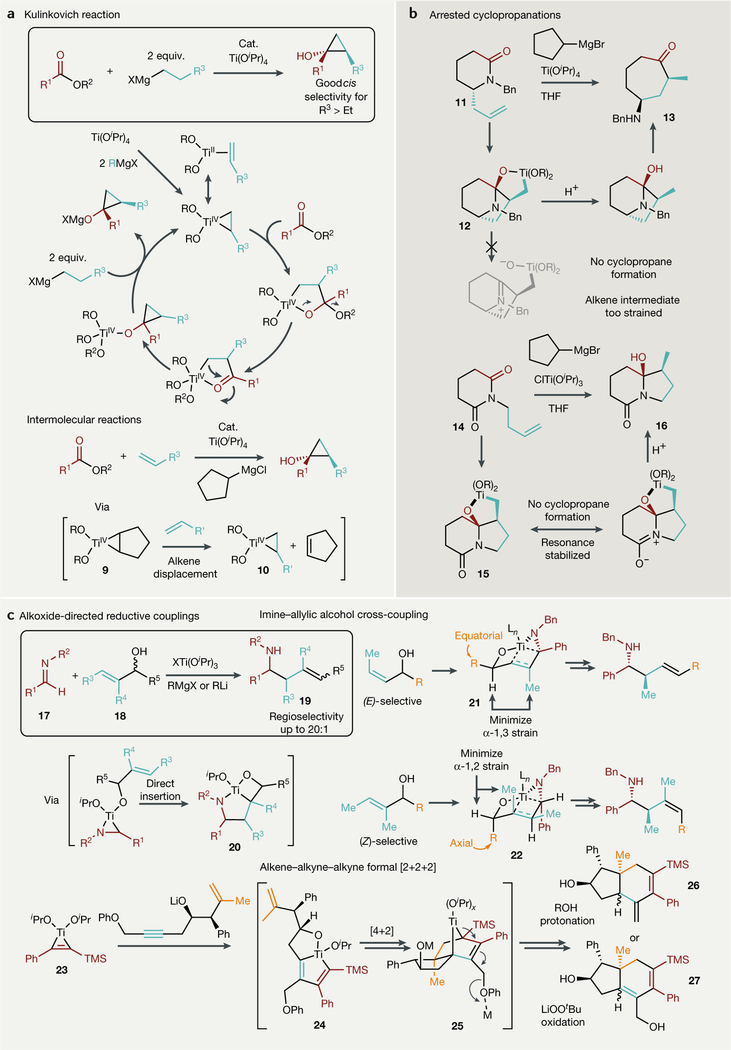
Fig. 3 |. Recent advances in two-electron reductive coupling reactions proceeding through titanacyclopropane and titanacyclopropene intermediates from Ti(OiPr)4/RM and related species.
a | Catalytic KuLinkovich cydopropanation reactions, in which ester C = O bond insertion into the Ti bond precedes pericycLic metaLLacycLe collapse. b | Stoichiometric arrested KuLinkovich-de Meijere-Like cyclopropanations, in which metaLLacycLe collapse is prevented through steric (top) or electronic (bottom) blocking. c | Alkoxide-directed regiospecific and stereospecific reductive couplings that bias regiospecific metaLLacycLe formation and subsequent insertion steps through covalent Ti-O bonding. DCE, dichLoroethane; DME, dimethoxyethane; equiv., equivalents; THF, tetrahydrofuran; TMS, trimethyLsiLyL.