Fig. 6 |. Single-electron processes catalysed by TiIII complexes.
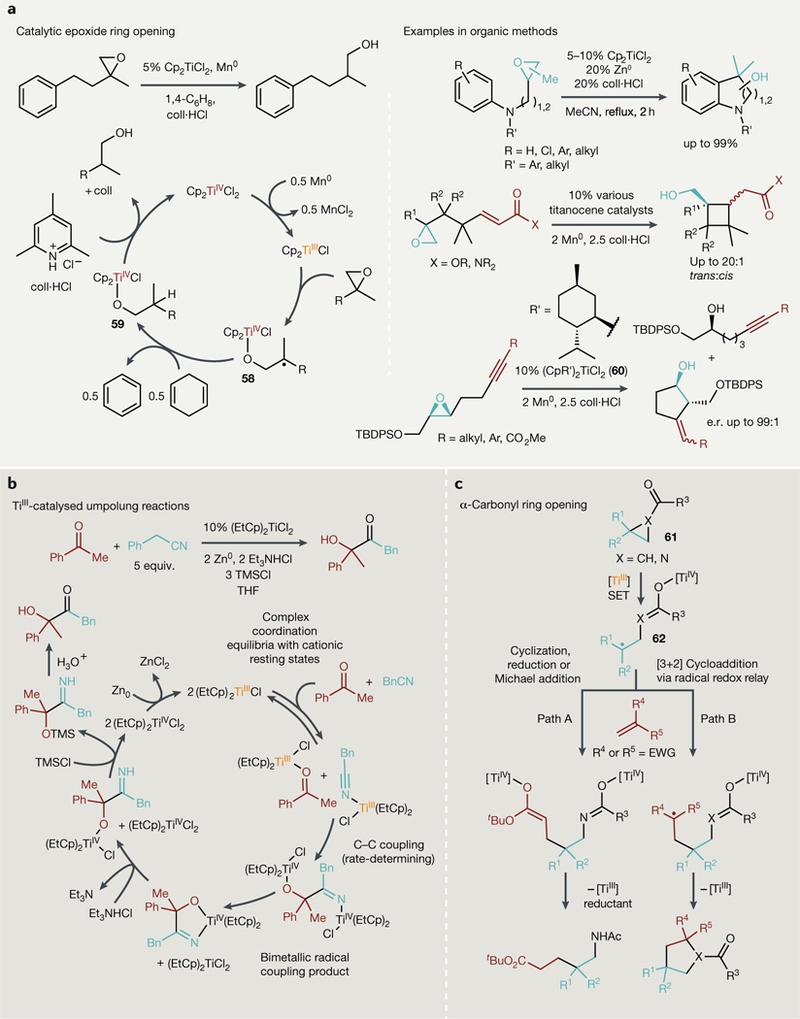
a | Catalytic epoxide ring opening using weak reductants and acids to drive turnover (Left) and examples in selective organic synthesis. b | Single-electron umpolung reactions can yield new C-C bonded products through a complex bimetallic radical coupling mechanism. c | SingLe-eLectron transfer (SET) from TiIII into carbonyls adjacent to strained rings gives access to several new aLkene coupling reactions including cyclization, Michael addition and [3+2] cycLoadditions. CoLL-HCL, 2,4,6-coLLidine hydrochloride; equiv, equivalents; e.r., enantiomeric ratio; EWG, eLectron-withdrawing group; TBDPS, tert-butyLdiphenylsiLyL; THF, tetrahydrofuran; TMS, trimethyLsiLyL.
