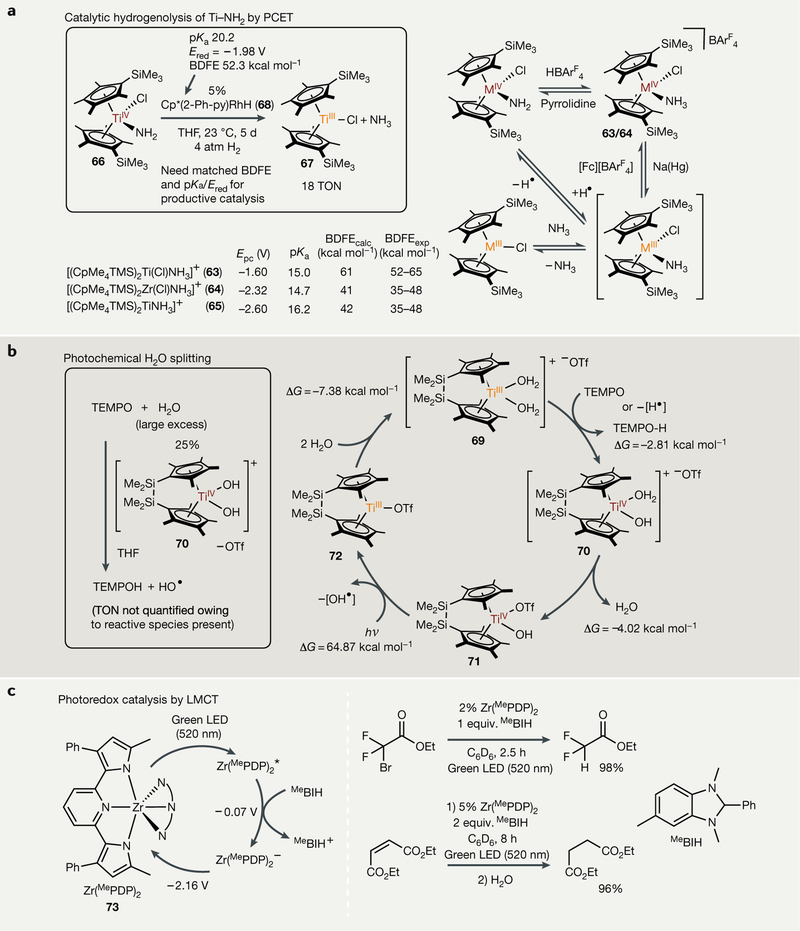
Fig. 7 |. Single-electron strategies in small-molecule activation chemistry.
a | Hydrogenoiysis of metal amides to ammonia via proton-coupled electron transfer (PCET), enabled by coordination-induced weakening of the N-H bond. b | Photochemical model of H2O splitting with TiIII catalysts, again enabled by coordination-induced weakening of O-H bonds. c | Photoredox catalysis via ligand-to-metal charge transfer (LMCT) with (MePDP)2Zr complexes, in which charge transfer occurs in a direction opposite of state-of-the-art late transition metal photoredox catalysts, which undergo metal-to-ligand charge transfer (MLCT). hv, irradiation; BDFE, bond dissociation free energy; Cp*, C5Me5−; Epc, cathodic peak potential; equiv, equivalents; Ered, reduction potential; Fc, ferrocene; LED, light-emitting diode; MePDP, 2,6-bis (5-methyl-3-phenyl-1H-pyrrol-2-yl)-pyridine; TEMPO, (2,2,6,6-tetramethy[piperidin-1-y[)oxy[; THF, tetrahydrofuran; TMS, trimethylsilyL; TON, turnover number.