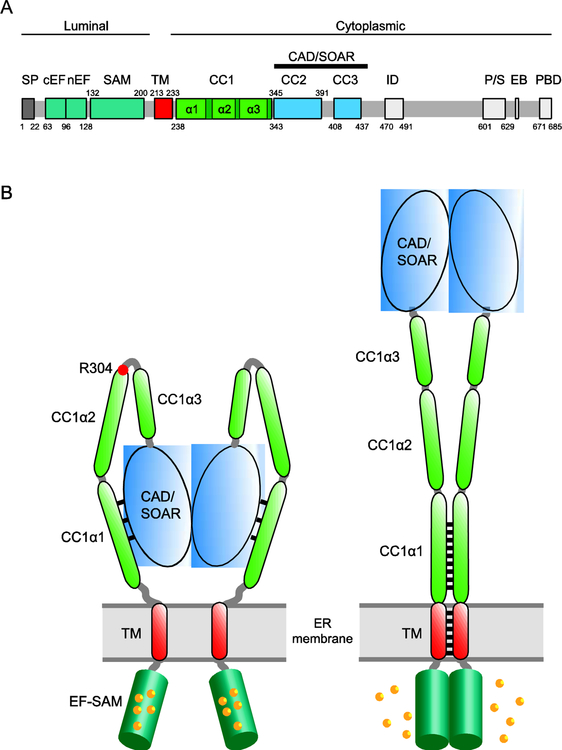
Figure 2. The initial events of STIM1 activation.
A. Domain organization of STIM1. Colored regions indicate structural and functional domains (SP, signal peptide; cEF, canonical EF hand; nEF, noncanonical EF hand; SAM, sterile alpha motif; TM, transmembrane domain; CC1–3, putative coiled-coil domains 1–3; CAD, CRAC activation domain (aa 342–448) or SOAR, STIM-Orai activating region (aa 344–442); ID, inactivation domain; P/S, proline/serine-rich domain; EB, EB1 binding domain; PBD, polybasic domain. B. Cartoon showing STIM1 activation by ER Ca2+ depletion. In the resting state (left), the luminal EF-SAM domains are bound with 5–6 Ca2+ and separated. The cytosolic domain is in a compact conformation in which CC1α1 binds to CAD/SOAR and keeps it close to the ER membrane. Following store depletion (right), Ca2+ release from the EF-SAM domain triggers its dimerization which brings the TM domains together to form a coiled-coil. The rearrangement dissociates CC1α1 from CAD/SOAR to extend the coiled-coil (represented by black lines) and move CAD/SOAR towards the PM. The affinity and number of Ca2+ ions released may help explain the [Ca2+]ER sensitivity and high cooperativity of STIM1 and Orai1 activation in vivo (K1/2 ~200 μM, Hill coefficient of 4–8; [6,60]).