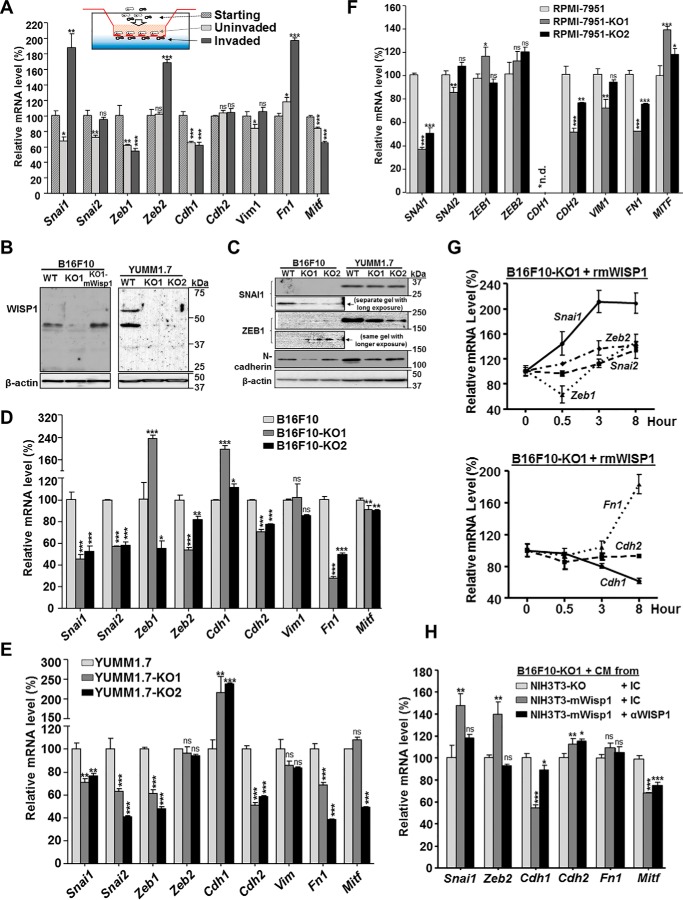
Figure 5.
WISP1 induced an EMT gene signature in mouse/human melanoma cells. Unless otherwise specified, all cells were plated on 6-well plates in complete growth medium for 48 h before they were harvested for RNA analysis or treated with the indicated conditioned medium or recombinant protein. A, mRNA expression, revealed by real-time quantitative RT-PCR, of select EMT marker genes and Mitf in uninvaded and invaded B16F10 cells from a Boyden transwell invasion assay. B, immunoblot analysis of WISP1 protein to confirm the disruption of Wisp1 gene in B16F10 and YUMM1.7 knockout cells. 20 μg of whole-cell lysate was loaded in each lane, and β-actin was used as an internal loading control. B16F10-KO1-mWisp1 cells, in which mouse WISP1 expression was resumed with retroviral transduction, were used as a positive control. C, immunoblot analysis of certain EMT marker proteins in B16F10 and YUMM1.7 knockout cells. 20 μg of whole-cell lysate was loaded in each lane, and all cells were compared on the same gel to reveal the relative intensity of each protein. D, comparison of EMT marker gene expression in mouse melanoma B16F10 and its two Wisp1-knockout cells (-KO1 and -KO2). E, comparison of EMT marker gene expression in mouse melanoma YUMM1.7 and its two Wisp1-knockout cells (-KO1 and -KO2). F, comparison of EMT marker gene expression in human melanoma RPMI-7951 and its two WISP1-knockout cells (-KO1 and -KO2). G, stimulation of EMT marker gene expression with recombinant mouse WISP1 protein (rmWISP1). B16F10-KO1 cells were treated with rmWISP1 (final concentration 5 μg/ml) and harvested at the indicated time point for real-time quantitative RT-PCR analysis. H, stimulation of EMT marker gene expression with WISP1-overexpressed or WISP1-immunodepleted conditioned medium (CM). The conditioned media were pretreated with the indicated antibodies for 30 min before they were used on Wisp1-knockout B16F10 cells (-KO1). The cells were collected for real-time qRT-PCR after 3 h of treatment. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant. Error bars, S.D.