Figure 1.
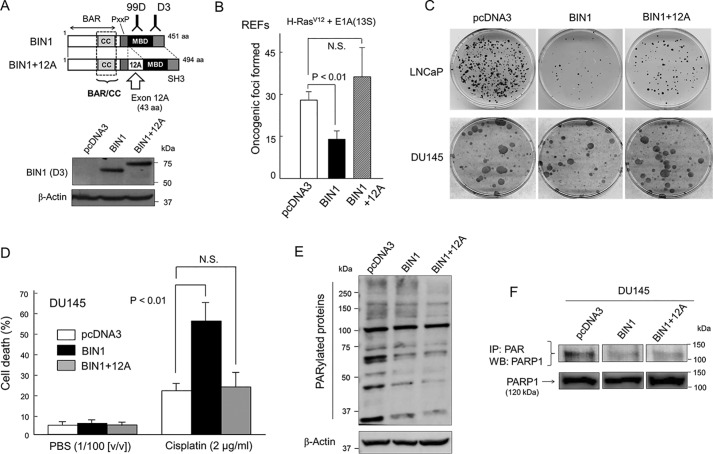
BIN1 and BIN1 + 12A decrease cellular PARylation, but only BIN1 inhibits cancer cell proliferation and increases cisplatin sensitivity. A, schematic diagrams of BIN1 and BIN1 + 12A proteins. The BIN1 exon 12A mRNA, which encodes 43 amino acids (aa) derived from the brain-specific BIN1 mRNA (which is also known as amphiphysin II) (27, 28, 31), is aberrantly incorporated by alternative splicing in several advanced cancers (31). Western blot analysis probed with an anti-BIN1 (clone D3) mAb detected both BIN1 and BIN1 + 12A proteins. BAR, BIN1/amphiphysin/Rvs-homologous domain (27, 28); BAR/CC, a BAR coiled-coil domain, which encodes the BIN1 effector domain (33–35). PxxP, a putative SH3-interacting domain (P is proline and x is any aa); MBD, MYC-binding domain; SH3, Src homology domain 3 (27, 28). B, primary REFs were transformed by cotransfection of two oncogenes, c-H-RasG12V and adenoviral E1A(13S) genes (27). Oncogenic foci were stained with Giemsa's solution and scored (27, 35). N.S. means (statistically) not significant. C, colony formation assays demonstrated that ectopically expressed BIN1 inhibited formation of G418-resistant colonies in LNCaP (a castration-sensitive prostate cancer cell line) but not in DU145 (a castration-resistant prostate cancer cell line). However, BIN1 + 12A transfection failed to suppress the colony-forming activity regardless of the cell lines. G418-resistant colonies were stained with Giemsa's solution and scored (33). D, at 24 h post-transfection with the indicated vectors, growing DU145 cells were treated with cisplatin (2.0 μg/ml) or phosphate-buffered saline (PBS) (1:100 (v/v)) for 72 h and were subjected to the trypan blue exclusion assays. E, Western blot analysis probed with an anti-poly(ADP-ribosyl)ated (PARylated) carbohydrate antibody (anti-PAR antibody) revealed reduced cellular PARylation after overexpression of BIN1 or BIN1 + 12A proteins in plain DU145 cells, which naturally express abundant PARP1 (34). The pcDNA3 empty vector was used as the negative control. F, immunoprecipitation probed with an anti-PAR antibody followed by Western blot analysis with an anti-PARP1 antibody demonstrated the inhibitory effect of overexpressed BIN1 and BIN1 + 12A proteins on the self-PARylation (i.e. automodification) of PARP1 in DU145 cell lysates.