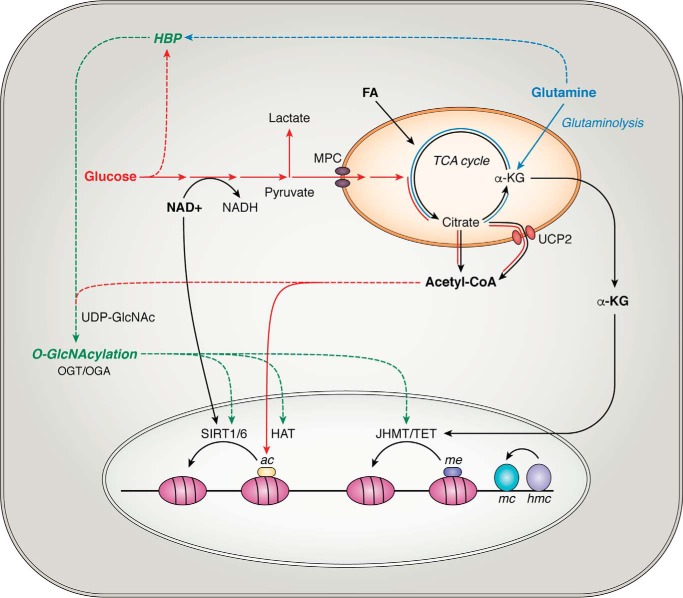
Figure 2.
Influence of metabolism on the epigenome in the maintenance of pluripotency. Glycolytic flux regulates the NAD+/NADH ratio, which controls the activities of sirtuin (SIRT) histone deacetylases. TCA cycle intermediates exported from the mitochondria include citrate and α-KG. Conversion of cytosolic citrate to Ac-CoA provides a donor for HAT-mediated histone acetylation. α-KG is a cofactor for histone and DNA demethylation reactions by JHDM and TET enzymes, respectively. By-products of glucose and glutamine catabolism supply reactants for O-GlcNAc modifications of epigenetic remodeling proteins through the HBP. Highlighted in red, blue, and green are the metabolic pathways associated with pluripotency, their contribution to TCA cycle metabolite production, and their subsequent influence on the epigenome (solid lines). Another potential mechanism for how metabolism can shape transcriptional patterns could be by fueling HBP-derived O-GlcNAcylation of proteins driving epigenome remodeling (dashed line).