Abstract
Background
To determine the additive value of quantitative radiomic texture features in predicting progression in human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinoma (OPSCC) based on pre-treatment CT.
Methods
Retrospective analysis of a single-center cohort of adult patients enrolled in a response-adapted radiation volume de-escalation trial treated with induction chemotherapy. Texture analysis of HPV-positive OPSCC was performed via primary tumor site contouring on pre-treatment contrast-enhanced CT scans. Percent change in size of the tumor in response to induction chemotherapy based on RECIST 1.1 criteria and progression free survival were clinically determined for this cohort. Receiver operating characteristic (ROC) analysis was performed to compare the accuracy of percent change in tumor size after induction chemotherapy with a combination of change in tumor size and radiomic texture features for predicting tumor progression.
Results
Radiomic texture analysis of the primary tumors in 38 patients with OPSCC depicted on pre-treatment neck CT scans using skewness and entropy in combination with percent change in tumor size after induction chemotherapy yielded a statistically significant increase in accuracy for predicting tumor progression over change in tumor size alone, with an area under the curve of 0.80 versus 0.56 (one-tailed P=0.0087).
Conclusions
This pilot study suggests that disease progression in patients with HPV-positive OPSCC is more accurately predicted using a combination of texture features on pre-treatment CT scans, along with change in tumor size compared to change in tumor size alone and could therefore serve as a radiomic texture signature.
Keywords: Computerized tomography (CT), texture analysis, oropharyngeal, cancer, size
Introduction
Human papillomavirus (HPV) is estimated to represent the most common cause of oropharyngeal squamous cell carcinoma (OPSCC) in the United States (1). Compared to HPV-negative OPSCC, HPV-positive OPSCC has better prognosis (2,3). However, some HPV-positive OPSCCs may display aggressive behavior leading to poor outcomes (4). The ability to predict the prognosis of HPV-positive OPSCC is of clinical significance because treatments may be tailored to optimize treatment of individual patients’ tumors. For example, early identification or prediction of poor responders could potentially avoid unnecessary drug toxicity and cost, and allow for the selection of alternative treatment regimens that could improve clinical outcome.
Certain molecular biomarkers in HPV-positive tumors seem to correlate with prognosis. For example, a class of oropharyngeal cancers with high p16 and lower p53 and Rb expression is associated with improved prognosis (4). Otherwise, HPV status alone was found to be of no prognostic value for local recurrence (4). Ultimately, biopsy samples represent a small fraction of the tumors and especially in the case of heterogeneous tumors; the tissue in the biopsy specimen may not accurately represent the entire tumor, thereby limiting the usefulness of some biomarkers (5).
Alternatively, radiomic texture analysis may serve as a non-invasive whole-tumor biomarker for oropharyngeal squamous cell carcinomas. For example, several CT texture features exhibit significant differences between HPV-positive and HPV-negative oropharyngeal squamous cell carcinomas, suggesting that these texture parameters may be predictive of HPV status in oropharyngeal squamous cell carcinomas (6-8). Another study found that MRI texture analysis could successfully predict p53 status of head and neck squamous cell carcinomas, a biomarker of tumor aggressiveness and poor prognosis, with 81% accuracy (9). Furthermore, primary tumor CT texture and histogram analysis parameters were found to be associated with overall survival in patients with locally advanced squamous cell carcinoma of the head and neck treated with induction chemotherapy (10). Similarly, the overexpression of VEGF was positively associated with the increased heterogeneity on pre-treatment 18FDG-PET for pharyngeal cancers and represents an independent prognostic predictor (11,12).
The goal of this study is to determine the additive value of radiomic texture features of the HPV-positive primary oropharyngeal squamous cell carcinoma in predicting progression-free survival.
Methods
Patients
This retrospective study was approved by institutional review board and informed consent was waived. Patients with locally advanced HPV-positive oropharyngeal squamous cell carcinoma who participated in a clinical trial from May 2010 to March 2014 were included in this study (13). All the primary oropharyngeal tumors included in the trial were biopsied and HPV status was determined at study enrollment by either p16 immunohistochemistry or in situ hybridization. Furthermore, eligible patients were at least 18 years of age with histologically proven and measurable stage IVa or IVb tumors, a Karnofsky performance scale index for assessment of functional impairment of at least 70% (14), and normal organ and marrow function. Exclusion criteria included metastatic disease, symptomatic peripheral neuropathy, prior chemotherapy or radiation therapy, or current immunosuppressive therapy. Two cycles of induction chemotherapy were administered every 21 days.
Patients with measurable locally advanced tumor received two cycles of induction chemotherapy (cisplatin, paclitaxel, cetuximab ± everolimus). Response was evaluated radiographically via contrast-enhanced neck CT after completion of induction chemotherapy by a neuroradiologist. Good response (GR) was defined as ≥50% reduction in the sum of target measurements as defined by RECIST 1.1. Patients with <50% decrease in the sum of target lesions were classified as non-responders (NR). Patients with ≥50% reduction in the sum of tumor diameters, or good response, received TFHX (paclitaxel, fluorouracil, hydroxyurea, and 1.5 Gy twice daily RT every other week) to a dose of 75 Gy with the single planning target volume (PTV1) encompassing exclusively gross disease and created by a uniform expansion of the gross tumor volume (GTV) by 1.5 cm to a dose of 75 Gy. Intensity-modulated radiation therapy (IMRT) was used with image guidance.
Patients with <50% response (non-response) were treated with TFHX encompassing the planning target volume (PTV2) and the next nodal station at risk to a dose of 45 Gy followed by a sequential boost to the planning target volume (PTV1) to a dose of 75 Gy. Patients were also evaluated via CT of the neck and chest at 1, 6, 12, and 24 months after chemoradiotherapy. There was improved late toxicity from RT volume de-escalation, including a lower rate of G-tube dependency at 1-year after. However, acute toxicity was not significantly different between patients with GR versus NR, although acute toxicity is also attributable to chemotherapy, which was consistent among patients with GR versus NR (13).
Progression status was determined based on clinico-radiological assessment and disease progression was defined based on RECIST 1.1. Biopsy or surgery was only performed in patients believed to have residual disease after treatment based on clinicoradiological assessment. The time to progression was defined as the period between the date of the initial CT and date of progression. Patients with ≥50% reduction in the sum of tumor diameters, or good response, received TFHX (paclitaxel, fluorouracil, hydroxyurea, and 1.5 Gy twice daily RT every other week) to a dose of 75 Gy with the single planning target volume encompassing exclusively gross disease. Patients with <50% response (non-response) were treated with TFHX encompassing the planning target volume and the next nodal station at risk to a dose of 45 Gy followed by a sequential boost to the planning target volume to a dose of 75 Gy.
Kaplan-Meier analysis
The relationships between tumor heterogeneity, progression, and response to induction chemotherapy were also assessed with Kaplan-Meier analysis to ascertain how the texture features correlate with progression-free survival. Progression free survival was defined as the length of time during and after the treatment that a patient lives with the disease, but does not worsen. Kaplan-Meier curves for progression free survival and differences between survival curves were determined by a non-parametric log rank test. A P of less than 0.05 was considered significant.
CT acquisition
Pre-treatment contrast-enhanced neck CT scans obtained at our institution of patients with measurable oropharyngeal squamous cell carcinomas were included in the analysis, as part of the response-adapted volume de-escalation clinical trial (13). The images were acquired on multidetector Philips Brilliance 64 slice CT scanners after intravenous injection of nonionic iodinated contrast medium (350 mg of iodine per milliliter, Omnipaque) at a rate of 1.2 mL/s and 55 s delay after the start of the injection. The scan parameters included 120 kV; 250 mAs; rotation time, 1.0 second; pitch, 0.75; collimation, 24 mm × 1.2 mm with a B30s smoothing algorithm; section thickness, 3 mm; intervals, 3 mm, and display field of view, 20 to 25 cm.
CT texture analysis
A quantitative in-house developed Radiomics Texture Analysis (RTA) workstation was used to compute texture features within each contoured region directly from the HU units within the image data, yielding radiomic features of the magnitude and spatial variations that describe the heterogeneity of the image pattern (15). Scans with tumors at least partly obscured by dental amalgam artifact were excluded from the analysis. Segmentations of the primary lesion on pre-treatment CT scans (Figure 1) were manually performed by a researcher under the supervision of a fellowship-trained neuroradiologist with CAQ and 5 years of experience, both of whom were blinded to the clinical outcomes. A single slice through the central portion of the tumor was selected for texture analysis in order to avoid volume averaging effects. The regions of interest did not extend beyond the margins of the tumors in order to avoid including normal surrounding tissues and air.
Figure 1.
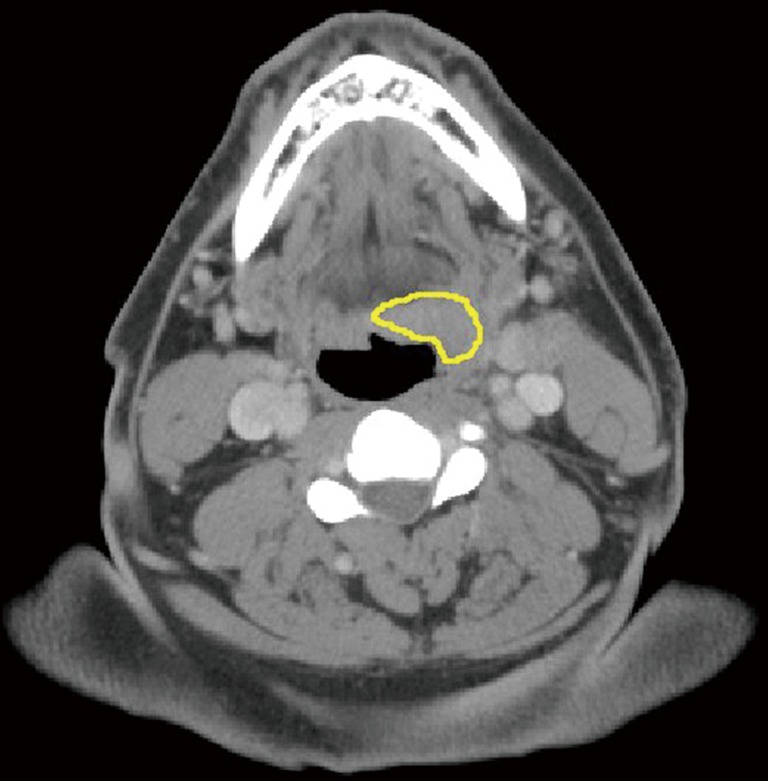
Screen capture of the University of Chicago Radiomics Texture Analysis workstation shows an ROI outlining a left oropharyngeal squamous cell carcinoma.
The following texture features were calculated: contrast, correlation, difference entropy, difference variance, energy, entropy, homogeneity, information measure of correlation 1 and 2, maximum correlation coefficient, sum average, sum entropy, sum variance, variance, and skewness. Note that these texture features can be described by two categories: (I) one feature group based on intensity (magnitude) distributions within the ROI and (II) another feature group based on spatial (pattern) distributions within the ROI. The highest performing texture features from the intensity-based and the spatial variation groups were chosen to be merged with the percent change in tumor size based on RECIST 1.1 using round robin linear discriminant analysis (LDA), which resulted in a linear weighted sum of the features, i.e., a texture signature (16). Skewness and entropy were selected for inclusion in the texture signature by applying stepwise feature selection. Stepwise feature selection was iteratively performed in a leave-one-out manner, and the two most frequently selected features were retained for the final model.
The resulting radiomic texture features characterizing the magnitude and spatial variation of the HU pattern within the oropharyngeal tumors were skewness and entropy, respectively. Skewness can be expressed mathematically by equation 1. In this equation, x represents each pixel value, µ is the mean of pixel values, and σ is the standard deviation of pixel values:
| [1] |
On the other hand, entropy describes the level of randomness in an image. A high entropy value indicates a low degree of order, while a low entropy value indicates a high degree of order. In this expression, c is the gray-level co-occurrence matrix indexed by i and j:
| [2] |
ROC analysis & statistical evaluation
The accuracy of texture features extracted from the radiologist-assigned oropharyngeal carcinoma tumor key slice to predict progression was evaluated using ROC methodology with the area under the curve (AUC) serving as the metric of performance (17). The PROPROC software package developed at the University of Chicago was used to calculate the estimate of the AUC for each curve according to the proper binormal model (10). The AUC was calculated for the percent change in tumor size feature alone, and for the texture signature, which included the percent change in tumor size merged with the two texture features characterizing the magnitude and spatial variation of the HU pattern within the tumor. The statistical significance of the difference between the AUCs was assessed using the one-tailed P of the correlated area test statistic, and the 95% confidence interval for the difference. A P value of less than 0.05 was considered significant.
Results
A total of 38 patients (4 females, 34 males; average age 62.1 years) with HPV-positive oropharyngeal squamous cell carcinoma and available contrast-enhanced neck CTs underwent texture analysis. Among the total of 38 patients with HPV-positive oropharyngeal squamous cell carcinoma and available contrast-enhanced neck CTs, 19 patients that had good response to induction chemotherapy and 19 patients had no significant response to induction chemotherapy based on RECIST 1.1 criteria (Tables 1 and 2). Furthermore, there were 7 patients with disease progression and 31 patients without disease progression. The patients that responded to induction chemotherapy had significantly (P=0.036) better progression free survival than the patients that did not respond to induction chemotherapy (Figure 2). The median follow up time was 41 months.
Table 1. Patient characteristics for tumor response to induction chemotherapy.
| Characteristic | GR (N=19) | PR (N=19) | P |
|---|---|---|---|
| Median age in years [range] | 61 [54–77] | 62 [46–79] | 0.61 |
| Male, n (%) | 17 (89.5) | 17 (89.5) | 1.00 |
| Tumor stage ≥ T3, n (%) | 11 (57.9) | 9 (47.4) | 0.52 |
| Tumor stage ≥ N2b, n (%) | 17 (89.5) | 17 (89.5) | 1.00 |
GR, good response; PR, poor response; DP, disease progression; NP, no progression.
Table 2. Patient characteristics for disease progression.
| Characteristic | DP (N=7) | NP (N=31) | P |
|---|---|---|---|
| Median age in years [range] | 66 [59–79] | 61 [46–77] | 0.85 |
| Male, n (%) | 7 (100.0) | 27 (87.1) | 0.32 |
| Tumor stage ≥ T3, n (%) | 5 (71.4) | 13 (41.9) | 0.16 |
| Tumor stage ≥ N2b, n (%) | 6 (85.7) | 26 (83.9) | 0.91 |
GR, good response; PR, poor response; DP, disease progression; NP, no progression.
Figure 2.
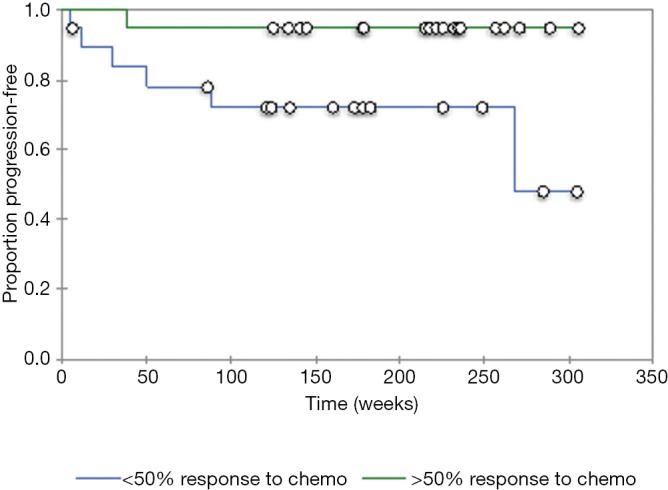
Kaplan-Meier curve of progression-free survival for tumors that had greater than 50% response to induction chemotherapy and those that had less than 50% response to induction chemotherapy (P=0.036).
The resulting texture feature statistics for patients with and without disease progression and the texture feature definitions are listed in Tables 3 and 4, respectively. The combination of radiographic texture analysis, including skewness and entropy, with percent change in size after induction chemotherapy yielded a statistically significant (one-tailed P=0.0087) increase in performance for assessing tumor progression over change in size alone, with an area under the ROC curve of 0.80 versus 0.56 (Figure 3). Tumors with radiomic texture signature greater than AUC of 0.80 trended towards decreased risk of progression, although this was not statistically significant with a P-value of 0.408 (Figure 4). Examples of tumors with different clinical outcomes and corresponding RTA output results are depicted on Figure 5.
Table 3. Texture feature statistics.
| Texture features | Progression | No progression | |||
|---|---|---|---|---|---|
| µ | σ | µ | σ | ||
| % Reduction | 48.82 | 16.00 | 53.99 | 26.19 | |
| Contrast | 26.58 | 13.12 | 29.73 | 16.79 | |
| Correlation | 0.81 | 0.07 | 0.81 | 0.08 | |
| Difference Entropy | 2.26 | 0.51 | 2.37 | 0.33 | |
| Difference variance | 11.39 | 4.68 | 12.42 | 6.20 | |
| Energy | 0.01 | 0.02 | 0.00 | 0.00 | |
| Entropy | 5.89 | 1.11 | 6.09 | 0.58 | |
| Homogeneity | 0.37 | 0.14 | 0.34 | 0.08 | |
| IMC1 | −0.22 | 0.06 | −0.21 | 0.06 | |
| IMC2 | 0.86 | 0.05 | 0.86 | 0.06 | |
| Maximum Correlation | 0.83 | 0.07 | 0.84 | 0.08 | |
| Sum Average | 80.01 | 22.26 | 77.09 | 14.12 | |
| Sum Entropy | 4.00 | 0.60 | 4.10 | 0.34 | |
| Sum Variance | 277.19 | 145.40 | 307.57 | 153.15 | |
| Variance | 75.94 | 38.30 | 84.32 | 40.87 | |
| Skewness | −1.24 | 2.97 | −0.51 | 0.91 | |
µ, mean; σ, standard deviation.
Table 4. Texture feature definitions.
| Feature name | Equation |
|---|---|
| Contrast | |
| Correlation | |
| Difference entropy | |
| Difference variance | |
| Energy | |
| Entropy | |
| Homogeneity | |
| IMC1 | |
| IMC2 | |
| Maximum correlation coefficient | |
| Sum average | |
| Sum entropy | |
| Sum variance | |
| Skewness |
Notation: p(i,j): (i,j)th entry in a normalized gray-tone spatial-dependence matrix; px(i): ith entry in the marginal-probability matrix obtained by summing the rows of p(i,j); Ng: Number of distinct gray levels in the quantized image; py(j): ; px+y(k): ; px-y(k): .
Figure 3.
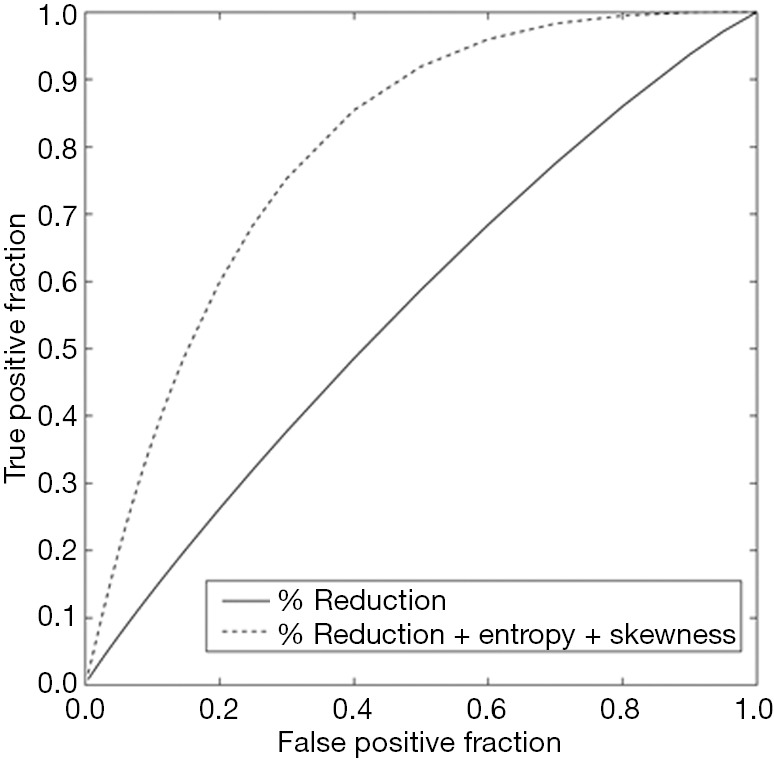
ROC plots demonstrate the improved performance for predicting progression free survival when change in tumor size is combined with texture features to yield a tumor signature.
Figure 4.
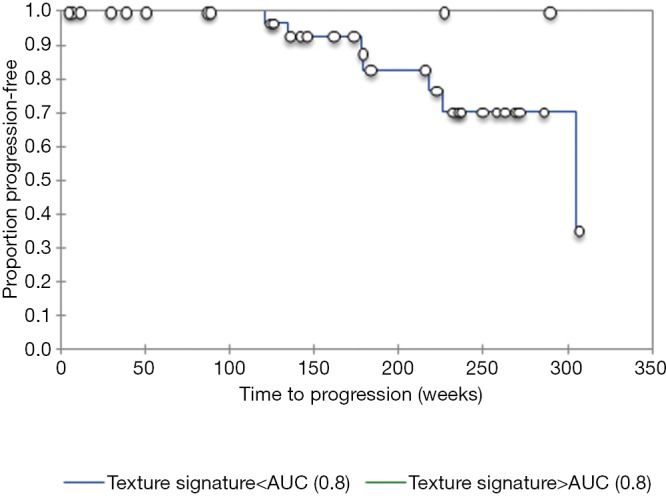
Kaplan-Meier survival curveshows that tumors with radiomic texture signature greater than AUC of 0.80 trended towards decreased risk of progression.
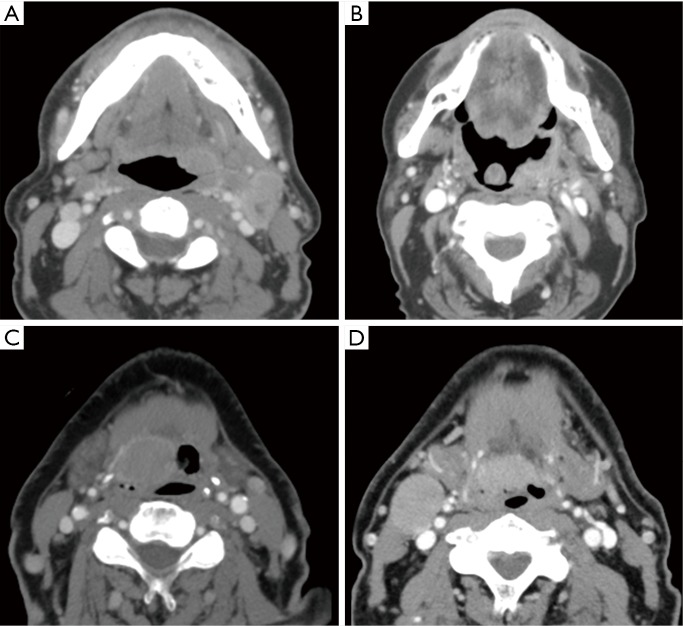
Figure 5.
Examples of CT images of oropharyngeal tumors in four different patients with their corresponding status in terms of progression versus no progression and the Radiomics Texture Analysis output result. (A) Progression correctly identified as progression with a high likelihood value of 3.63, (B) progression incorrectly identified as non-progression with a low likelihood value of 0.03, (C) no progression correctly identified as non-progression with a low likelihood value of −0.54, (D) and no progression incorrectly identified as progression with a high likelihood value of 1.05.
Discussion
There is a trend towards personalization of oncologic therapy in head and neck oncology, with the goal of optimizing treatment outcomes for individual patients (18). With respect to radiological imaging, this can be accomplished through the emerging field of radiomics, which consists of a high-throughput extraction of advanced quantitative features in images with the use of mathematical algorithms. These features contain information about tumor shape, tumor intensity, and tumor texture, and are often beyond the discerning capabilities of the human visual system (19). In particular, texture analysis quantitatively characterizes the spatial distribution of pixels and gray-level intensities in radiological images of a region of interest (20).
A large number of radiomic features have been found to have prognostic power in independent data sets for various types of cancers (21,22). In particular, tumor spatial heterogeneity is an important prognostic factor (8,23). Indeed, a radiomic signature based on shape, intensity, texture, and wavelet transform showed that head and neck squamous cell carcinomas that are more heterogeneous on CT tend to have worse local control (8). Furthermore, higher entropy and positive skewness tend to correlate with increased heterogeneity and portend poorer prognosis, as was found in this study for HPV-positive oropharyngeal squamous cell carcinomas and several other types of tumors (19). Similarly, certain CT histogram and gray-level run-length features of the primary tumors in patients with head and neck squamous cell carcinoma are associated with local failure in patients with head and neck squamous cell carcinoma treated with chemoradiotherapy (24). Ultimately, CT radiomics has the potential for assessing the risk of specific tumor outcomes using multiple stratification groups in patients with head and neck cancer (18).
The basis for the prognostic value of a radiomic signature that reflects intratumor heterogeneity may be associated with underlying gene-expression patterns (21,25). Thus, it is not surprising that the combination of skewness and entropy calculated on the central slice pre-treatment CT of the HPV-positive oropharyngeal squamous cell carcinomas in this study showed additive prognostic value over percent size change alone in assessing progression post induction chemotherapy. This pertains to predicting the response of tumors to radiotherapy prior to the start of treatment could enhance clinical care management by enabling the personalization of treatment plans based on predicted outcome. Indeed, applying texture analysis to routine clinical imaging can potentially serve as an opportunity to improve decision-support in cancer treatment at low cost (21).
Although the results of this study were statistically significant, the patient cohort was relatively small, which precluded the use of independent datasets for training and validation. Instead, we used a round-robin approach for the evaluation. Nevertheless, there were few confounding effects in this study. For example, the addition of Everolimus to the induction chemotherapy regimen was not found to be beneficial (13). Furthermore, the elimination of elective nodal coverage in patients with good response to induction chemotherapy did not appear to compromise outcomes. Also, the use of a single representative slice of CT for the texture analysis did not allow for evaluation of volumetric features that could provide a more representative measure of tumor heterogeneity. This may be the subject of future studies. There was no normalization of images prior to texture analysis. However, the scans were acquired with identical protocols and processed in a similar fashion in order to minimize any variation in tissue enhancement. This theoretical limitation may be addressed in future studies on the subject. Ultimately, the prognosis of patients included in the particular clinical trial for this study may or may not be relevant to other treatment regimens or subset of tumors of the head and neck cancer.
With regards to image quality, CT scans with dental amalgam streak artifact and other CT artifacts at the level of the tumors were excluded from the analysis in this study. Although some of the results in this study were statistically significant, the patient cohort was relatively small, which precluded the use of independent datasets for training and validation (26).
Conclusions
In this preliminary study, a radiomic signature that consists of percent change in primary tumor size combined with skewness and entropy of HPV-positive oropharyngeal squamous cell carcinoma on pre-treatment CT was shown to yield a statistically significant improved performance in the task of predicting progression free survival over percent change in tumor size alone. However, further validation of this finding in a larger cohort is warranted.
Acknowledgments
This study was in part funded by the RSNA medical student research grant. The clinical database utilized in this study was provided by James Melotek MD, MS.
Ethical Statement: This retrospective study was approved by institutional review board and informed consent was waived.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Stein AP, Saha S, Kraninger JL, Swick AD, Yu M, Lambert PF, Kimple RJ. Prevalence of Human Papillomavirus in Oropharyngeal Cancer: A Systematic Review. Cancer J 2015;21:138-46. 10.1097/PPO.0000000000000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang MB, Liu IY, Gornbein JA, Nguyen CT. HPV-Positive Oropharyngeal Carcinoma A Systematic Review of Treatment and Prognosis. Otolaryngol Head Neck Surg 2015;153:758-69. 10.1177/0194599815592157 [DOI] [PubMed] [Google Scholar]
- 3.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved Survival of Patients With Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J Natl Cancer Inst 2008;100:261-9. 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 4.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A. Molecular Classification Identifies a Subset of Human Papillomavirus-Associated Oropharyngeal Cancers With Favorable Prognosis. J Clin Oncol 2006;24:736-47. 10.1200/JCO.2004.00.3335 [DOI] [PubMed] [Google Scholar]
- 5.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. N Engl J Med 2012;366:883-92. 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita A, Buch K, Li B, Kawashima Y, Qureshi MM, Sakai O. Difference Between HPV-Positive and HPV-Negative Non-Oropharyngeal Head and Neck Cancer: Texture Analysis Features on CT. J Comput Assist Tomogr 2016;40:43-7. 10.1097/RCT.0000000000000320 [DOI] [PubMed] [Google Scholar]
- 7.Buch K, Fujita A, Li B, Kawashima Y, Qureshi MM, Sakai O. Using Texture Analysis to Determine Human Papillomavirus Status of Oropharyngeal Squamous Cell Carcinomas on CT. AJNR Am J Neuroradiol 2015;36:1343-8. 10.3174/ajnr.A4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogowicz M, Riesterer O, Ikenberg K, Stieb S, Moch H, Studer G, Guckenberger M, Tanadini-Lang S. Computed Tomography Radiomics Predicts HPV Status and Local Tumor Control After Definitive Radiochemotherapy in Head and Neck Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys 2017;99:921-8. 10.1016/j.ijrobp.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Graham CM, Elci O, Griswold ME, Zhang X, Khan MA, Pitman K, Caudell JJ, Hamilton RD, Ganeshan B, Smith AD. Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 2013;269:801-9. 10.1148/radiol.13130110 [DOI] [PubMed] [Google Scholar]
- 10.Dang M, Lysack JT, Wu T, Matthews TW, Chandarana SP, Brockton NT, Bose P, Bansal G, Cheng H, Mitchell JR, Dort JC. MRI Texture Analysis Predicts p53 Status in Head and Neck Squamous Cell Carcinoma. AJNR Am J Neuroradiol 2015;36:166-70. 10.3174/ajnr.A4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SW, Shen WC, Lin YC, Chen RY, Hsieh TC, Yen KY, Kao CH. Correlation of pretreatment 18F-FDG PET tumor textural features with gene+ expression in pharyngeal cancer and implications for radiotherapy-based treatment outcomes. Eur J Nucl Med Mol Imaging 2017;44:567-80. 10.1007/s00259-016-3580-5 [DOI] [PubMed] [Google Scholar]
- 12.Cheng NM, Fang YH, Chang JT, Huang CG, Tsan DL, Ng SH, Wang HM, Lin CY, Liao CT, Yen TC. Textural features of pretreatment 18F-FDG PET/CT images: prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J Nucl Med 2013;54:1703-9. 10.2967/jnumed.112.119289 [DOI] [PubMed] [Google Scholar]
- 13.Villaflor VM, Melotek JM, Karrison TG, Brisson RJ, Blair EA, Portugal L, De Souza JA, Ginat DT, Stenson KM, Langerman A, Kocherginsky M, Spiotto MT, Hannigan N, Seiwert TY, Cohen EE, Vokes EE, Haraf DJ. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol 2016;27:908-13. 10.1093/annonc/mdw051 [DOI] [PubMed] [Google Scholar]
- 14.Karnofsky DA, Burchenal JH. In: Evaluation of chemotherapeutic agents. MacLeod CM, editor. New York: Columbia University Press; 1949. The clinical evaluation of chemotherapeutic agents in cancer; pp. 191-205. [Google Scholar]
- 15.Chinander MR, Giger ML, Martell JM, Favus MJ. Computerized radiographic texture measures for characterizing bone strength: A simulated clinical setup using femoral neck specimens. Med Phys 1999;26:2295-300. 10.1118/1.598743 [DOI] [PubMed] [Google Scholar]
- 16.Johnson RA, Wichern DW. Applied multivariate statistical analysis 3rd ed. Englewood Cliffs, NJ: Prentice-Hall, 1992. [Google Scholar]
- 17.Metz CE. Basic Principles of ROC Analysis. Semin Nucl Med 1978;8:283-98. 10.1016/S0001-2998(78)80014-2 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann TK, Schuler PJ, Laban S, Grässlin R, Beer M, Beer AJ, Friebe-Hoffmann U, Bullinger L, Möller P, Wiegel T. Response Evaluation in Head and Neck Oncology: Definition and Prediction. ORL J Otorhinolaryngol Relat Spec 2017;79:14-23. 10.1159/000455726 [DOI] [PubMed] [Google Scholar]
- 19.Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, Ganeshan B, Miles KA, Cook GJ, Goh V. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 2012;3:573-89. 10.1007/s13244-012-0196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol 2004;59:1061-9. 10.1016/j.crad.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 21.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014;5:4006. 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parmar C, Leijenaar RT, Grossmann P, Rios Velazquez E, Bussink J, Rietveld D, Rietbergen MM, Haibe-Kains B, Lambin P, Aerts HJ. Radiomic feature clusters and prognostic signatures specific for Lung and Head & Neck cancer. Sci Rep 2015;5:11044. 10.1038/srep11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raja JV, Khan M, Ramachandra VK, Al-Kadi O. Texture analysis of CT images in the characterization of oral cancers involving buccal mucosa. Dentomaxillofac Radiol 2012;41:475-80. 10.1259/dmfr/83345935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuno H, Qureshi MM, Chapman MN1, Li B, Andreu-Arasa VC, Onoue K, Truong MT, Sakai O. CT Texture Analysis Potentially Predicts Local Failure in Head and Neck Squamous Cell Carcinoma Treated with Chemoradiotherapy. AJNR Am J Neuroradiol 2017;38:2334-40. 10.3174/ajnr.A5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Li H, Guo W, Drukker K, Lan L, Giger ML, Ji Y. Deciphering Genomic Underpinnings of Quantitative MRI-based Radiomic Phenotypes of Invasive Breast Carcinoma. Sci Rep 2015;5:17787. 10.1038/srep17787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leijenaar RT, Carvalho S, Hoebers FJ, Aerts HJ, van Elmpt WJ, Huang SH, Chan B, Waldron JN, O'sullivan B, Lambin P. External validation of a prognostic CT-based radiomic signature in oropharyngeal squamous cell carcinoma. Acta Oncol 2015;54:1423-9. 10.3109/0284186X.2015.1061214 [DOI] [PubMed] [Google Scholar]