Surgery is an important treatment option for cancer patients. If all of the cancer cells can be surgically removed, the patient will be cured of that cancer (1,2). It is vital to obtain a negative tumor margin to achieve a successful cancer resection, as the presence of residual tumor cells after surgery is a major cause of tumor recurrence and leads to poor prognosis. Despite the extensive use of preoperative imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), surgical margin positivity rate has not been improved significantly over the past several decades. Margin positivity rates for all types of cancers can only reach 60% for the best and in some cases reach 15% (3,4). This is primarily because cancer surgery still highly relies on the visual inspection and palpation of the surgeons as well as intraoperative histopathological analysis of frozen tissue specimen. The former approach is very subjective, and the latter method is very time-consuming.
Finding an objective and straightforward approach to guide the surgical procedure and to define tumor margin is no doubt very beneficial for both the surgeons and the cancer patients. In this endeavor, fluorescence-guided surgery (FGS) has attracted a lot of attention in the surgical oncological field. FGS is an intraoperative medical technique used to generate a real-time fluorescence image of the surgical region and guide the surgical procedure. Compared to conventional imaging techniques such as CT and MRI, FGS can provide real-time imaging during surgery, and it is much cheaper and much easier to operate. The amount of FGS-related publications has increased in recent years. Figure 1 is a survey based on Google Scholar search results, showing that the publication number of FGS-related study in 1990 was close to zero and the number increased to more than 850 in 2018. In the following, we would like to provide a brief overview of the current status of FGS and then discuss some perspectives about the future development of FGS. We also use FGS for liver tumors as a showcasing example to demonstrate the potential power of FGS in surgical oncology.
Figure 1.
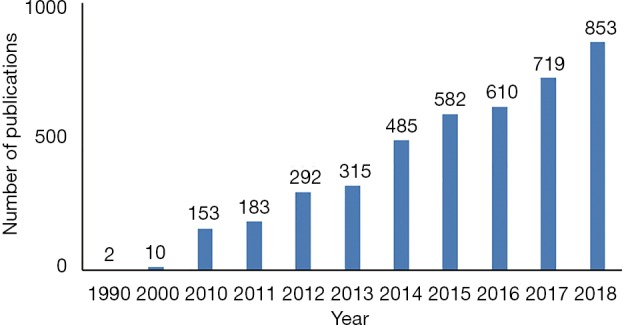
The FGS related publications during the periods of 1990–2018. FGS, fluorescence-guided surgery.
Technically, FGS requires two essential elements to operate, a fluorescence probe and an imaging device (3,5,6). The fluorescence probe is a chemical entity that absorbs light of a specific wavelength and emits light of a different, typically longer, wavelength. It is usually an organic molecule like a dye and can also be a biomacromolecule like a fluorescent protein or nanomaterials like quantum dots. Furthermore, to be suitable for FGS applications, the fluorescence probe needs to be able to accumulate in cancerous tissues. Though many fluorescent probes may fulfill the criteria mentioned above, the United States Food and Drug Administration (FDA) so far only approved a minimal number of fluorescence probes for clinical purpose. Table 1 lists four commonly used probes in clinical studies and their structures and excitation (Ex) and emission (EM) wavelength, as well as photophysical properties (3). They are indocyanine green (ICG), methylene blue (MB), fluorescein sodium, and 5-Aminolevulinic acid (5-ALA). Among them, ICG is the most widely used probe in FGS. For example, a recent survey shows that in about 100 clinical FGS studies, 60% of studies utilized ICG as the fluorescence probe (3). ICG is a water-soluble near-infrared (NIR) probe with a molecular weight of 776 Da (7). The excitation wavelength is at 780 nm, and the emission wavelength is at 820 nm. These two wavelengths are outside the range of most tissue autofluorescence. Also, NIR beam has excellent tissue penetration as compared with the visible laser. ICG is also known as its high safety index and low allergic reaction rate. MB and fluorescein sodium are used much less frequently, though fluorescein is, in fact, the first ever used fluorescence probe in history. 5-ALA is a unique probe as 5-ALA is not fluorescent by itself. 5-ALA can induce the synthesis and accumulation of the fluorescent molecule protoporphyrin IX (PpIX) in epithelial and neoplastic tissues (8,9).
Table 1. The Structures and properties of FDA approved fluorescence probes (3).
| FDA approved fluorescence probe | Molecular structure | Excitation wavelength (nm) | Emission wavelength(nm) |
|---|---|---|---|
| Indocyanine green (ICG) | 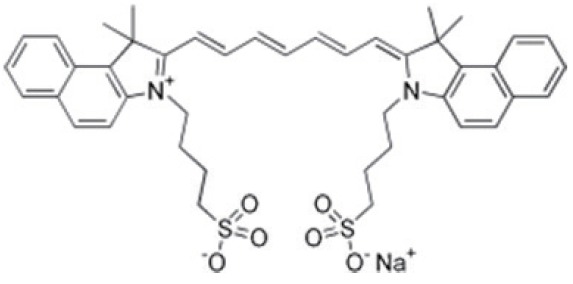 |
780 | 820 |
| Methylene blue (MB) | 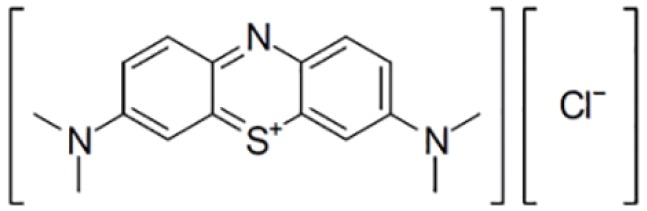 |
670 | 690 |
| Fluorescein sodium | 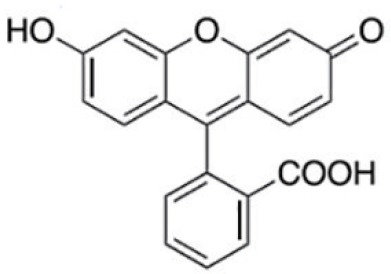 |
494 | 512 |
| 5-Aminolevulinic acid (5-ALA) | 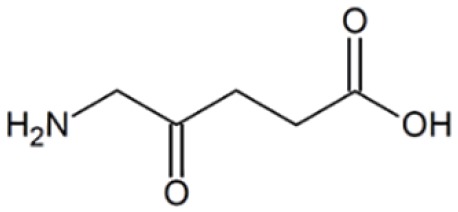 |
380–440 | 620 (alkaline pH); 634 (acidic pH) |
The imaging device is the other essential element in FGS. Though fluorescence imaging by itself can be considered as a mature optical technology, the FDA approved imaging systems for FGS are still limited. Experts in the field have pointed out that an ideal FGS system should possess the following six critical desirable features: (I) being able to overlay the white-light and fluorescence images in real time, (II) operation under ambient room lighting condition, (III) nanomolar-level fluorescence probe sensitivity, (IV) being able to analyze the image quantitatively, (V) simultaneous detection of multiple fluorophores in tissue, and (VI) maximized ergonomic use for open surgery (5). Several notable FGS manufacturer companies in today’s market are Novadaq Technologies, Hamamatsu Photonics, Fluoptics Minatec, Quest Medical Imaging, and VisionSense. These companies all have FDA approved systems to sell on the market. Among them, Novadaq Technologies is the leading manufacturer. The majority of FGS studies were conducted with Novadaq SPY system. The SPY system Novadaq utilized ICG as the fluorescence probe. It is the first FDA approved system and was FDA 510(k) cleared in 2005. New FGS systems according to the proposed essential, desirable features are being developed in recent years (3,5).
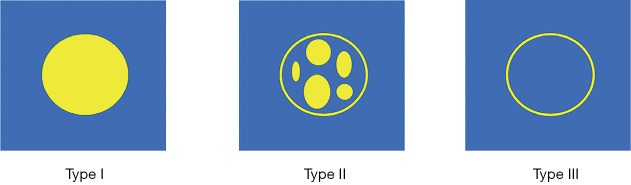
FGS so far has been used to treat a variety of cancers including head and neck cancer, breast cancer, lung cancer, esophagus cancer, hepatocellular carcinoma, gastric cancer, colorectal cancer, anal cancer, prostate cancer, penile cancer, and melanoma (3). We here would focus on its clinical use to treat liver tumor to showcase the power of FGS technique. First of all, a fascinating issue that we would like to point out is that the widely used FGS fluorescence probe, ICG, has been commonly used in clinical settings to estimate hepatic function since its FDA approval in 1954 (7,10). Such use can date back to 1954. Later, surgeons began to use ICG to image and visualize hepatobiliary structures. In recent years, ICG-based FGS technique begins to emerge as a useful tool in liver tumor surgery. Scientists have found that ICG can accumulate in the cancerous tissues in the liver and displays some unique fluorescence image patterns depending on liver cancer differentiation. As shown in Figure 2, the fluorescence patterns of liver tumors could be classified into three types. Type I indicates relatively uniform fluorescence distribution throughout the whole tumor region. Type II shows inhomogeneous fluorescence distribution throughout the tumor region. Type III is characterized by rim fluorescence, in which cancer tissues are negative for fluorescence, but the area surrounding the tumor is positive for fluorescence. Type I pattern is closely related to all well-differentiated hepatocellular carcinoma. In contrast, type III pattern is closely associated with poorly differentiated hepatocellular carcinoma and colorectal liver metastasis (10). Such information will be beneficial for the doctors to guide surgical procedure and to predict prognosis. Surgeons also find FGS is very useful when visual inspection and palpation are limited, for example, when locating subcapsular lesions during liver surgery with a laparoscope. Furthermore, FGS is very useful for liver segmentation. Liver segmentation is essential during liver surgery to better reserve liver function. Identifying hepatic boundary in many cases can be very challenging even for experienced surgeons. FGS could offer a better guide than conventional tools (10,11).
Figure 2.
Fluorescence imaging patterns of liver cancers with various differentiation.
The past decade no doubt has witnessed significant progress in the clinical application and technical development of FGS. There are still many rooms for further advancements on the FGS technology. The first perspective is about the new developments for the fluorescence probe. Though ICG is widely used as fluorescence probe in FGS field, its performance in tumor detectability is not very good and varies significantly from case to case. For example, in liver tumor treatment, the tumor detectability can range from 100% to 67% (10). This is likely because ICG is a passive tumor-targeted probe. Now smart tumor-targeted probes like enzyme/microenvironment-activated probes and receptor-targeted probes are emerging in the field (6). FGS with currently available probes is still suffering from background noise interference. The targeted smart probe may eventually solve this drawback. These smart probes will possess a recognition moiety in its structure and can bind onto cancer-specific receptor and could potentially lead to a significant increase of the surgical removal of cancer. Another type of novel targeted probes is a sprayable targeted probe. These types of probes can make the clinical use of the probes very convenient. It can be applied onto the region of interest by a simple spray, avoiding system or oral administration. The second perspective is about the further development of an imaging device. We envision that for the high-end product on the FGS market, integration of artificial intelligence (AI) to an FGS system can be an attractive direction to explore. An AI-empowered FGS system could help the surgeon to make an objective and standardized surgical decision. We also envision that for the low-end product on the FGS market, a portable and low-cost system would be very beneficial for the low-income countries or regions. Currently, a commercial FGS system could cost more than $200,000. An FGS system could be built using cheap parts with a cost as low as $3,200 with decent performance (12). The third perspective is about the integration of complementary analytical tools to further improve the decision-making capability of FGS in the surgical removal of cancer. FGS currently is not the silver bullet to determine the surgical margin of cancer. If other analytical tools can work together with FGS, the rate of positive margin may be reduced significantly. For example, a scientist can combine FGS with mass spectrometry (MS) (13), FTIR spectroscopy, and Raman spectroscopy (14) to further empower FGS. In the future, we may see MS-guided surgery, FTIR-guided surgery, and Raman-guided surgery as merging tools in cancer treatment. The integration of these analytical techniques may eventually lead to precision surgery in cancer treatment.
Acknowledgements
Funding: This study was supported by grants from the Beijing Municipal Science & Technology Commission research fund (No. Z171100000417004) and the National Natural Science Foundation of China (31500818).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation--a new cutting edge. Nat Rev Cancer 2013;13:653-62. 10.1038/nrc3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung TT. Superior oncological outcome in laparoscopic hepatectomy for hepatocellular carcinoma, hype or hope? Hepatobiliary Surg Nutr 2017;6:437-8. 10.21037/hbsn.2017.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagaya T, Nakamura YA, Choyke PL, et al. Fluorescence-Guided Surgery. Front Oncol 2017;7:314. 10.3389/fonc.2017.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moris D, Burkhart RA, Beal EW, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: are oncologic outcomes truly superior to an open approach? Hepatobiliary Surg Nutr 2017;6:200-2. 10.21037/hbsn.2017.03.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DSouza AV , Lin H, Henderson ER, et al. Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J Biomed Opt 2016;21:80901. 10.1117/1.JBO.21.8.080901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low PS, Singhal S, Srinivasarao M. Fluorescence-guided surgery of cancer: applications, tools and perspectives. Curr Opin Chem Biol 2018;45:64-72. 10.1016/j.cbpa.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 7.Deja M, Ahlers O, Macguill M, et al. Changes in hepatic blood flow during whole body hyperthermia. Int J Hyperthermia 2010;26:95-100. 10.3109/02656730903250574 [DOI] [PubMed] [Google Scholar]
- 8.Colditz MJ, Jeffree RL. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 1: Clinical, radiological and pathological studies. J Clin Neurosci 2012;19:1471-4. 10.1016/j.jocn.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 9.Montcel B, Mahieu-Williame L, Armoiry X, et al. Two-peaked 5-ALA-induced PpIX fluorescence emission spectrum distinguishes glioblastomas from low grade gliomas and infiltrative component of glioblastomas. Biomed Opt Express 2013;4:548-58. 10.1364/BOE.4.000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakaseko Y, Ishizawa T, Saiura A. Fluorescence-guided surgery for liver tumors. J Surg Oncol 2018;118:324-31. 10.1002/jso.25128 [DOI] [PubMed] [Google Scholar]
- 11.Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr 2016;5:322-8. 10.21037/hbsn.2015.10.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okusanya OT, Madajewski B, Segal E, et al. Small portable interchangeable imager of fluorescence for fluorescence guided surgery and research. Technol Cancer Res Treat 2015;14:213-20. 10.7785/tcrt.2012.500400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kröger S, Niehoff AC, Jeibmann A, et al. Complementary molecular and elemental mass-spectrometric imaging of human brain tumors resected by fluorescence-guided surgery. Anal Chem 2018;90:12253-60. 10.1021/acs.analchem.8b03516 [DOI] [PubMed] [Google Scholar]
- 14.Lane LA, Xue R, Nie S. Emergence of two near-infrared windows for in vivo and intraoperative SERS. Curr Opin Chem Biol 2018;45:95-103. 10.1016/j.cbpa.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]