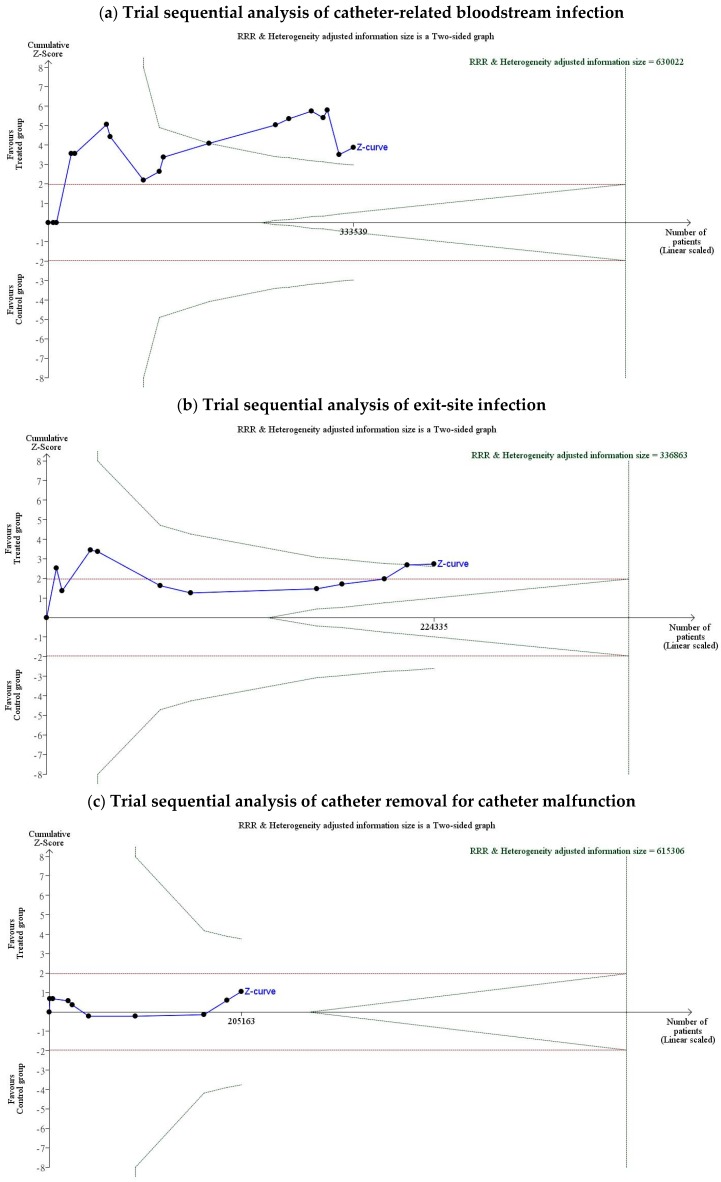
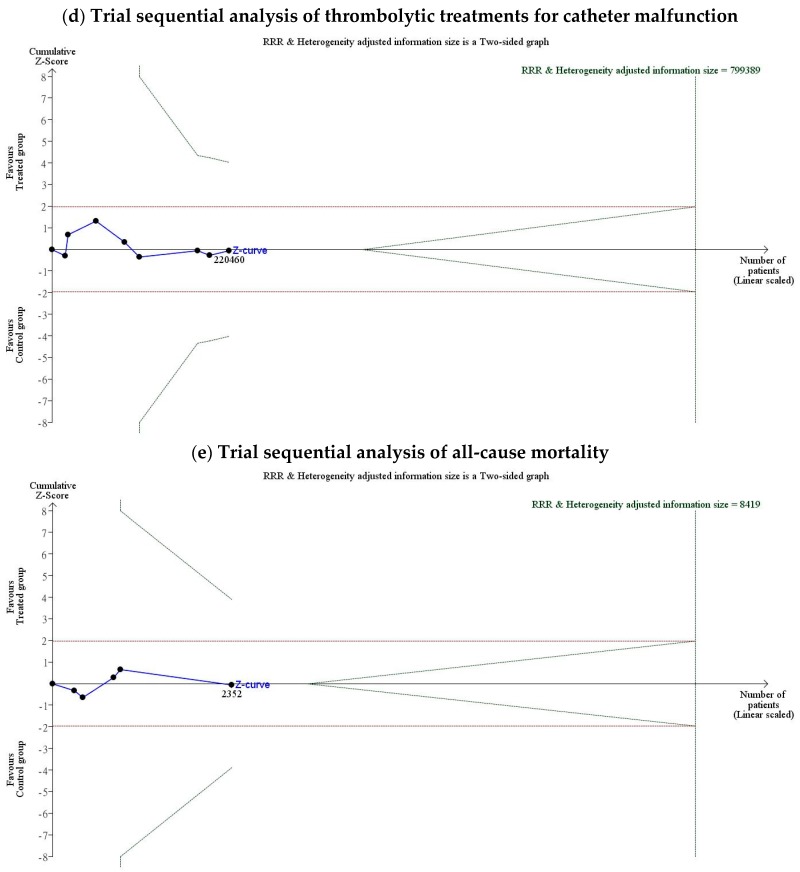
Figure 7.
Trial sequential analysis of the odds ratio for evaluation event: (a) Trial sequential analysis of catheter-related bloodstream infection. Trial sequential analysis of 17 studies with a lower risk of bias in reporting catheter-related bloodstream infection, with a control event proportion of 17%, diversity of 45%, type I error of 5%, power of 80%, and relative risk reduction of 30%. The required information size of 630,022 was not reached and none of the boundaries for benefit, harm, or futility were crossed, leaving the meta-analysis inconclusive at a 30% relative risk reduction. The overall OR of CRBSI was 0.439 (95% CI, 0.290–1.668; p < 0.001); (b) trial sequential analysis of exit-site infection. Trial sequential analysis of eleven studies with low risk of bias reporting exit-site infection, with a control event proportion of 17%, diversity of 30%, type I error of 5%, power of 80%, and relative risk reduction of 30%. The required information size of 336,863 was not reached and none of the boundaries for benefit, harm, or futility were crossed, leaving the meta-analysis inconclusive at a 30% relative risk reduction. The OR of ESI was 0.644 (95% CI, 0.469–0.883; p = 0.006); (c) trial sequential analysis of nine studies with a lower risk of bias reporting the need to remove the catheter for catheter malfunction, with a control event proportion of 17%, diversity of 71%, type I error of 5%, power of 80%, and relative risk reduction of 30%. The required information size of 625,306 were not reached and none of the boundaries for benefit, harm, or futility were crossed, leaving the meta-analysis inconclusive at a 30% relative risk reduction. The OR of the need to remove the catheter for catheter malfunction was 0.746 (95% CI, 0.431–1.293; p = 0.151); (d) trial sequential analysis of thrombolytic treatments for catheter malfunction. Trial sequential analysis of nine studies with low risk of bias reporting the need to receive thrombolytic treatment for catheter malfunction, with a control event proportion of 17%, diversity of 91%, type I error of 5%, power of 80%, and relative risk reduction of 30%. The required information size of 615,306 were not reached and none of the boundaries for benefit, harm, or futility were crossed, leaving the meta-analysis inconclusive at a 30% relative risk reduction. The OR of the need to receive thrombolytic treatment for catheter malfunction was 1.015 (95% CI, 0.655–1.573; p = 0.461); (e) trial sequential analysis of all-cause mortality. Trial sequential analysis of five studies with a lower risk of bias reporting all-cause mortality, with a control event proportion of 17%, diversity of 78%, type I error of 5%, power of 80%, and relative risk reduction of 30%. The required information size of 8419were not reached and none of the boundaries for benefit, harm, or futility were crossed, leaving the meta-analysis inconclusive at a 30% relative risk reduction. The OR of all-cause mortality was 0.976 (95% CI, 0.663–1.439; p = 0.296). Notes: The solid blue line is the cumulative Z-curve. The vertical black dashed line is required information size. The green dashed lines represent the trial sequential monitoring boundaries and the futility boundaries.