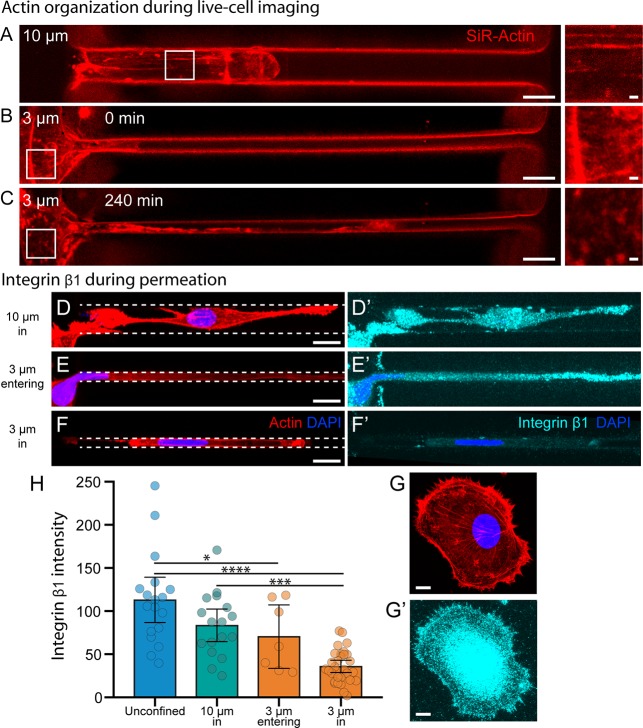
Figure 4.
Actin and integrin organization during microchannel permeation. (A–C) SiR-Actin live cell imaging of MDA-MB-231 breast cancer cells in 10 and 3 μm-wide microchannels. Strong F-actin organization is observed in 10 μm channels and at the periphery of 3 μm channels before permeation occurs (A and B, insets). After protruding over 100 μm into the narrow microchannel, the cytoskeleton at the periphery of the 3 μm channel is reorganized (C, inset). Scale bars = 10 μm, 1 μm in insets. (D–G) Maximum intensity projections of laser scanning confocal image stacks of (D–G) actin and (D′–F′) integrin β1 localization in fixed cells during various states of permeation. Unconfined (G′) and 10 μm channel (D′) cells exhibit strong integrin β1 localization at the cell–matrix interface. Cells exploring 3 μm channels (E′) exhibit similar patterns, but once cells are fully confined in the 3 μm channels (F′), integrin localization at the cell–matrix interface is greatly reduced. Scale bars = 10 μm. (H) The average integrin β1 intensity measured in saponin-permeabilized cells from all four conditions. N = 18, 17, 7, and 30. Error bars represent 95% confidence intervals. (* p < 0.05, *** p < 0.001, **** p < 0.0001. ANOVA, F = 18.08, DF = 71).