Figure 5. Cideb enhances SREBP processing by interacting with Sec12.
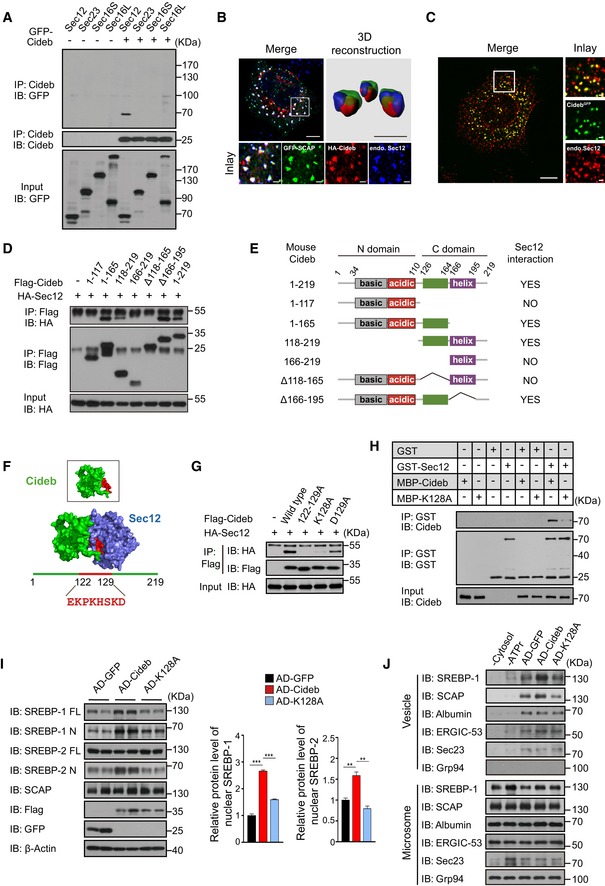
- Cideb interacts with Sec12. 293T cells transfected with Cideb and GFP‐tagged Sec12, Sec23, Sec16S, or Sec16L were subjected to immunoprecipitation with an anti‐Cideb antibody, and levels of the co‐immunoprecipitated protein were detected by an anti‐GFP antibody.
- Co‐localization of SCAP and Cideb at the ER exit sites. HepG2 cells transfected with GFP‐SCAP and HA‐Cideb were arrested with 10 μM BFA and 1 μM nocodazole for 2 h, then fixed with methanol, and stained with antibodies against Sec12 or HA. 3D reconstruction was obtained by processing 3D‐original image with Imaris. Scale bars represent 10 μm (Merge) and 1 μm (Inlay and 3D reconstruction).
- Localization of Cideb at the ER exit sites. AML12 cells knocked‐in with a GFP tag at the C‐terminus of Cideb genome were treated with 10 μM BFA for 1 h, then fixed with methanol, and stained with antibodies against Sec12. Scale bars represent 10 μm (Merge) and 1 μm (Inlay).
- Sec12 interacts with the 118–165 portion of Cideb. HA‐tagged Sec12 was co‐expressed with the indicated Flag‐tagged Cideb truncation mutants in 293T cells and immunoprecipitated with an anti‐Flag antibody. Levels of the co‐immunoprecipitated protein were detected by an anti‐HA antibody following SDS–PAGE.
- The schematic diagram of Cideb and its truncations.
- Sec12/Cideb interaction modeled by molecular dynamic simulation. The red portion highlighted the predicted domain of Cideb interacting with Sec12.
- Lysine 128 of Cideb is required for the interaction with Sec12. 293T cells transfected with HA‐Sec12 and the indicated Flag‐tagged Cideb constructs were subjected to immunoprecipitation with an anti‐Flag antibody, and levels of the co‐immunoprecipitated HA‐Sec12 were detected with an anti‐HA antibody.
- Recombinant Cideb interacts with Sec12. GST‐Sec12 and MBP‐tagged Cideb or Cideb‐K128A were purified from E. coli and subjected to in vitro binding.
- Cideb‐K128A fails to promote SREBP processing. Cideb −/− mice were injected with adenovirus expressing GFP, Flag‐Cideb, or Flag‐Cideb‐K128A, and sacrificed 7 days after injection. Liver lysates were subjected to IB with the indicated antibodies following SDS–PAGE and quantification of IB data from three independent experiments. Data represent Mean ± SEM; **P < 0.01; ***P < 0.001, by 2‐tailed Student's t‐test.
- Cideb, but not Cideb‐K128A, promotes SCAP/SREBP incorporation into COPII vesicles. Liver microsomes were isolated from mice expressing the indicated proteins and subjected to in vitro budding assay. Resulting vesicles and the donor microsomes were subject to IB with the indicated antibodies following SDS–PAGE.
Source data are available online for this figure.
