Figure 6. Disruption of homo-dimer does not significantly affect the enzyme activity of AtGCL.
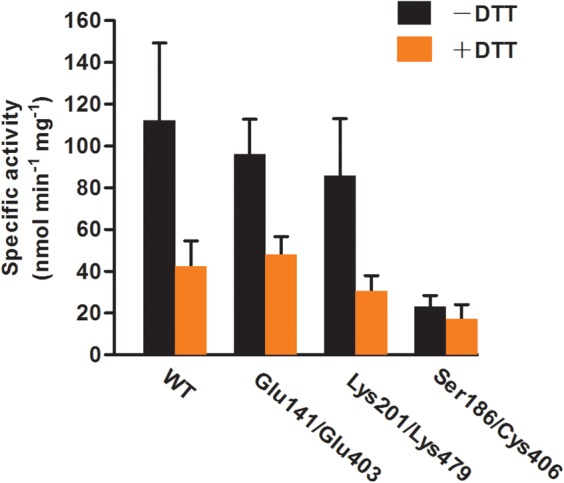
The statistical analysis (two-way ANOVA followed by the Holm–Šídák method) indicates that both factors, DTT treatment and enzyme type, have an impact on enzyme activities, but there is no significant interaction between two sources of variation (DTT treatment and enzyme type). With respect to the activity in the presence/absence of DTT, the dimer interface mutants Glu141/Glu403 and Lys201/Lys479 are not significantly different when compared with WT GCL, although oxidized mutant proteins do not dimerize (see Figure 4). The activity of the disulfide bridge mutant Ser186/Cys406 is not significantly affected by DTT as expected. Finteraction (3, 23) = 2.90, Pinteraction = 0.06; FDTT(1, 23) = 35.33, PDTT < 0.001; Fenzyme type(3, 23) = 10.57, Penzyme type < 0.001. Enzyme reactions were performed as described under Materials and methods section. Values for specific activities (nmol min−1 mg−1) are expressed as means ± SD (n > 3).
