Figure 7. Structure of the GSM–GCL complex confirms that in the dimeric state GSM has free access to the active site.
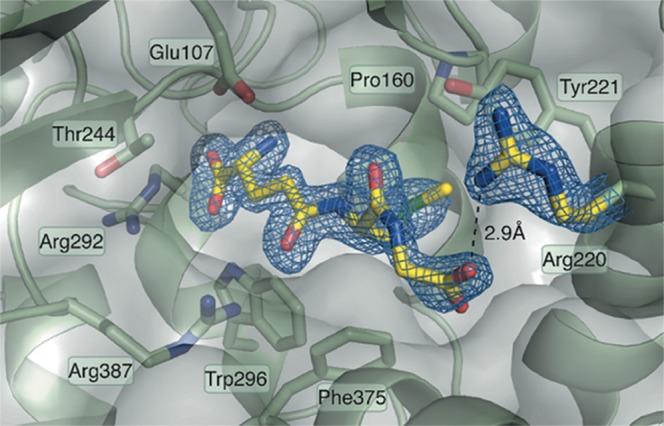
The well-defined density for GSM indicates the binding mode associated with feedback inhibition: the glutamate and cysteine parts of the GSH analog bind deep into the active site pocket, whereas the glycine moiety interacts with the conserved Arg220 at the solvent accessible end of the tunnel. GSM and GSH are therefore competitive inhibitors of GCL.
