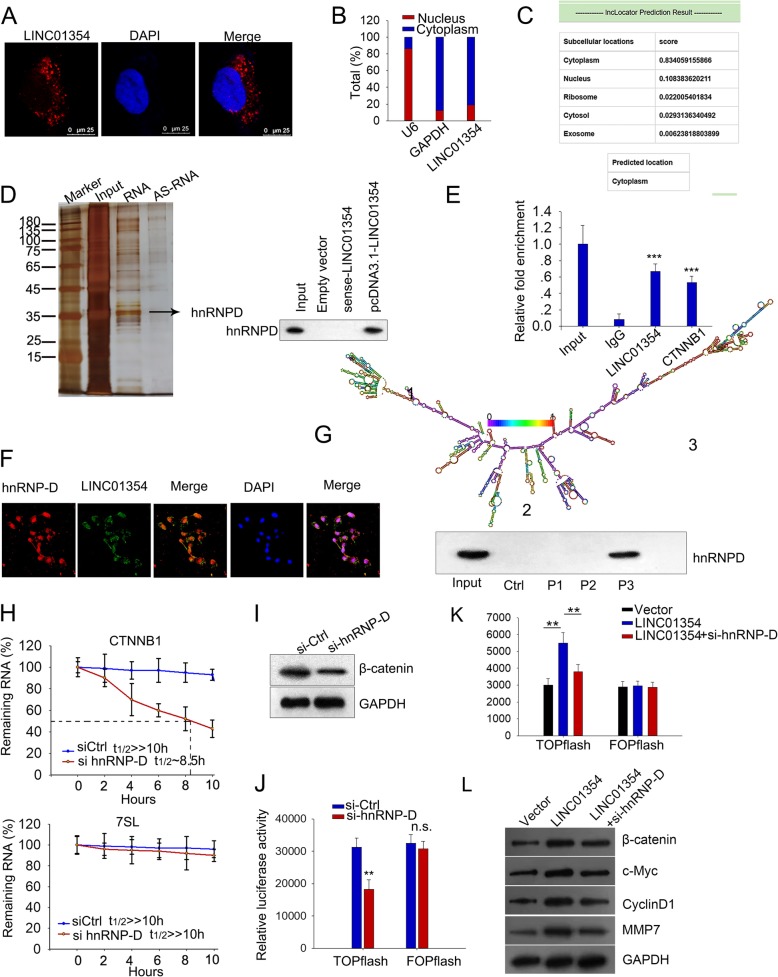
Fig. 7.
LINC01354 mediated the stabilization of CTNNB1 through interacting with RNA-binding protein hnRNP-D. a RNA FISH revealed the cytoplasm localization of LINC01354 in LoVo cells. b-c LINC01354 mainly presented in the cytoplasm by qRT-PCR analysis and such analysis was also verified on online. d hnRNP-D was identified among the potential binding partners with LINC01354 by RNA pull-down assays and mass spectrometry, and confirmed by western blotting. e RIP experiments in vitro exhibited a specific enrichment of LINC01354 and CTNNB1 with the hnRNP-D antibody but not IgG. f FISH assay demonstrated the overlapped location of LINC01354 and hnRNP-D expression in cytoplasm (g) The secondary structure of LINC01354 was presented and the fragment responsible for its interaction with hnRNP-D was identified by pulldown assay and western blot. h The stability of CTNNB1 could be decreased by silencing the level of hnRNP-D. i-j Knocking down hnRNP-D in LoVo cells, the level of β-catenin was significantly reduced as presented by western blot assay, and the Wnt/β-catenin was inactivated as depicted by TOP-Flash luciferase assay. k Silencing hnRNP-D abrogated the activation of overexpressing LINC01354 on Wnt/β-catenin as observed by TOP-Flash luciferase assay. l Western blot analysis showed that silencing hnRNP-D countervailed the inductive effect of overexpressing LINC01354 on the expression of β-catenin, c-Myc, CycinD1, and MMP7. Data were exhibited as the mean ± SD. All assays were carried out in triplicate. **p < 0.01 and ***p < 0.001