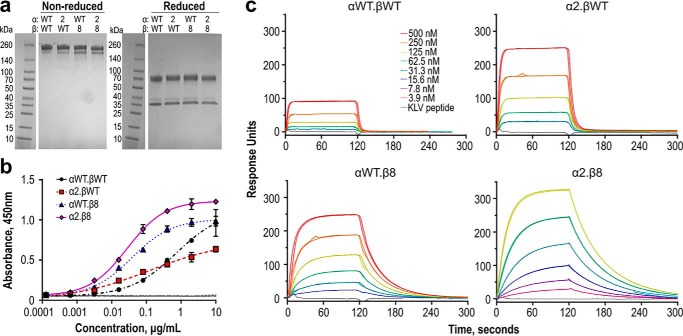
Figure 5.
Engineered RA14 TCR2ds–huFc variants show increased affinity for NLV/A2. a, TCR2ds–huFc formats of each variant were purified by protein A and size-exclusion chromatography to isolate only the intact protein. Protein purity was evaluated by nonreduced and reduced 4–20% gradient SDS-polyacrylamide gel (3 μg of protein per lane). b, tetramer-binding activities of purified RA14 variants were compared by ELISA. Plates were coated with NLV/A2 tetramer, followed by TCR2ds–huFc and goat anti-human Fc-HRP. Data shown are the average and range of duplicate series for a representative experiment; this was repeated several times with similar results. c, pMHC binding kinetics were measured by SPR. Each TCR2ds–huFc variant was immobilized on a CM5 chip at 2000–5000 RUs, after which monomeric NLV/A2 was injected at six concentrations between 3.9 and 500 nm. An in-line blank flow cell was used to assess background binding. Peptide specificity was evaluated with injections of monomeric KLV/A2 at the maximum concentration used for NLV/A2 for each variant. All injections were performed in duplicate; shown are the data and fits with the numerical values reported in Table 2.