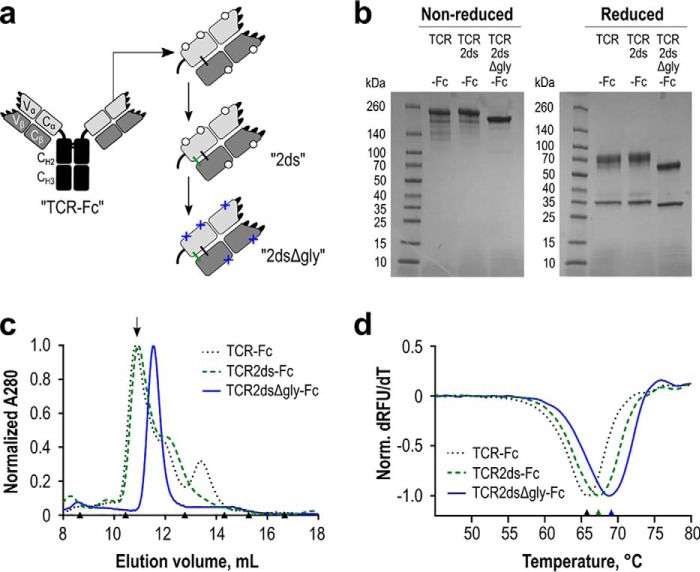
Figure 6.
Expression and stability of the WT RA14 TCR as a soluble Fc fusion protein. a, several iterations of the TCR-Fc fusion protein were designed. In all scaffolds, the TCR α-chain was fused to a human IgG1 core/lower hinge and Fc. Open circles represent native glycosylation sites. Additional modifications include a second disulfide bond resulting from inclusion of the upper hinge sequence (ds, green) in the TCR2ds–huFc format and the removal of predicted N-linked glycosylation sites (Δgly, blue crosses) in the TCR2dsΔgly-huFc format. b, purity of each protein A purified design was evaluated via reducing and nonreducing 4–20% gradient SDS-PAGE, with 3 μg loaded per lane. c, protein homogeneity was analyzed by size-exclusion chromatography. The arrow indicates the major peak collected for experiments using a glycosylated scaffold. Triangles indicate elution volumes for molecular weight standards: thyroglobulin with a 669-kDa size eluted at 8.7 ml; ferritin 440 kDa at 10.5 ml; aldolase 158 kDa at 12.8 ml; conalbumin 75 kDa at 14.3 ml; ovalbumin 44 kDa at 15.2 ml; carbonic anhydrase 29 kDa at 16.7 ml. Representative data are shown for each design. d, melting temperature of each design was analyzed by differential scanning fluorimetry. An average value for two separate normalized curves is shown for each scaffold from a representative experiment.