Figure 12.
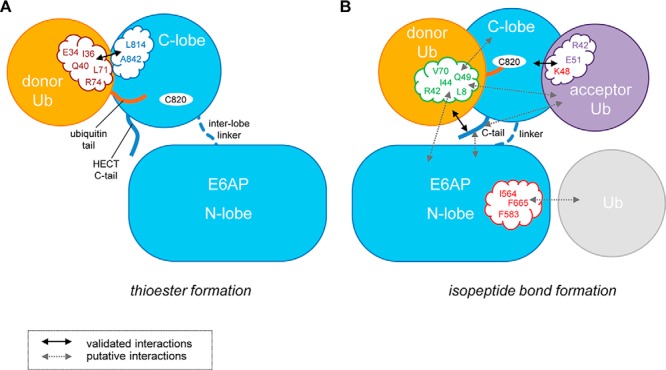
Schematic view summarizing the identified interactions and surface patches critical for Lys-48–linked ubiquitin chain formation by the HECT domain of E6AP. Our studies suggest that the C-lobe of E6AP utilizes canonical (NEDD4-type) contacts with the donor ubiquitin (rose) during thioester transfer of ubiquitin from the E2 to the E3. The C-terminal tail (C-tail) of E6AP is not required for this step (A). E6AP-mediated isopeptide bond formation between the thioester-linked donor–E6AP complex and an acceptor ubiquitin relies on surface regions that are distinct from those required during thioester transfer (B). The hydrophobic patch (green) is used by the donor ubiquitin for yet uncharacterized interactions with the N-lobe, the C-lobe, or the acceptor ubiquitin. The acceptor ubiquitin is critically dependent on a hydrophilic area (purple) around the acceptor residue (Lys-48), including Glu-51. The C-tail contacts the thioester-linked donor and confers linkage specificity in isopeptide bond formation, possibly by additional contacts with the N-lobe or the acceptor ubiquitin. Furthermore, we demonstrate that E6AP interacts with ubiquitin through the N-lobe. Mutations of residues in the exosite region (red) weaken this interaction and reduce isopeptide bond formation activity; however, they do not affect thioester formation. Whether the N-lobe–ubiquitin interaction resembles the exosite-mediated binding mode seen in NEDD4-type ligases awaits structural elucidation. It also remains unclear which functional ubiquitin moiety this interaction involves (e.g. a regulatory moiety or, possibly, the acceptor).
