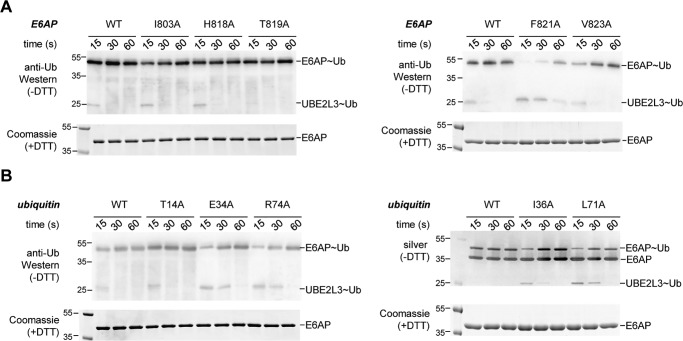
Figure 2.
Most of the residues identified by NMR are important for thioester transfer of ubiquitin from UBE2L3 to E6AP. A, thioester transfer of ubiquitin from the E2 (UBE2L3) to the E6AP HECT domain, followed in single-turnover, pulse-chase assays at three time points, as indicated, and monitored by nonreducing SDS-PAGE and anti-ubiquitin Western blotting. The thioester-linked HECT domain–ubiquitin conjugate (E6AP∼Ub) and, in some cases, the thioester-linked E2-ubiquitin precursor (UBE2L3∼Ub) are visible. The input amount of HECT domain (E6AP) is monitored by reducing SDS-PAGE and Coomassie staining. Note that no auto-ubiquitination of the HECT domain occurs within the tested time range. B, analogous assays as in A, monitoring the effect of mutations in ubiquitin on thioester transfer to the E6AP HECT domain. For the I36A and L71A variants, silver staining (right) was used in lieu of anti-ubiquitin Western blotting (left), because these variants are not detected well by the antibody (P4D1) used here.