Abstract
Background
Dementia is a chronic, progressive and ultimately fatal neurodegenerative disease. Advanced dementia is characterised by profound cognitive impairment, inability to communicate verbally and complete functional dependence. Usual care of people with advanced dementia is not underpinned universally by a palliative approach. Palliative care has focused traditionally on care of people with cancer but for more than a decade, there have been increased calls worldwide to extend palliative care services to include all people with life‐limiting illnesses in need of specialist care, including people with dementia.
Objectives
To assess the effect of palliative care interventions in advanced dementia and to report on the range of outcome measures used.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group's Specialized Register on 4 February 2016. ALOIS contains records of clinical trials identified from monthly searches of several major healthcare databases, trial registries and grey literature sources. We ran additional searches across MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), CINAHL (EBSCOhost), LILACS (BIREME), Web of Science Core Collection (ISI Web of Science), ClinicalTrials.gov and the World Health Organization ICTRP trial portal to ensure that the searches were as comprehensive and as up‐to‐date as possible.
Selection criteria
We searched for randomised (RCT) and non‐randomised controlled trials (nRCT), controlled before‐and‐after studies (CBA) and interrupted time series studies evaluating the impact of palliative care interventions for adults with dementia of any type, staged as advanced dementia by a recognised and validated tool. Participants could be people with advanced dementia, their family members, clinicians or paid care staff. We included clinical interventions and non‐clinical interventions. Comparators were usual care or another palliative care intervention. We did not exclude studies on the basis of outcomes measured and recorded all outcomes measured in included studies.
Data collection and analysis
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, when required, consulted with the rest of the review team. We independently extracted data and conducted assessment of methodological quality, using standard Cochrane methods.
Main results
We identified two studies of palliative care interventions for people with advanced dementia. We did not pool data due to the heterogeneity between the two trials in terms of the interventions and the settings. The two studies measured 31 different outcomes, yet they did not measure the same outcome. There are six ongoing studies that we expect to include in future versions of this review.
Both studies were at high risk of bias, in part because blinding was not possible. This and small sample sizes meant that the overall certainty of all the evidence was very low.
One individually randomised RCT (99 participants) evaluated the effect of a palliative care team for people with advanced dementia hospitalised for an acute illness. While this trial reported that a palliative care plan was more likely to be developed for participants in the intervention group (risk ratio (RR) 5.84, 95% confidence interval (CI) 1.37 to 25.02), the plan was only adopted for two participants, both in the intervention group, while in hospital. The palliative care plan was more likely to be available on discharge in the intervention group (RR 4.50, 95% CI 1.03 to 19.75). We found no evidence that the intervention affected mortality in hospital (RR 1.06, 95% CI 0.53 to 2.13), decisions to forgo cardiopulmonary resuscitation in hospital or the clinical care provided during hospital admission, but for the latter, event rates were low and the results were associated with a lot of uncertainty.
One cluster RCT (256 participants, each enrolled with a family carer) evaluated the effect of a decision aid on end‐of‐life feeding options on surrogate decision‐makers of nursing home residents with advanced dementia. Data for 90 participants (35% of the original study) met the definition of advanced dementia for this review and were re‐analysed for the purposes of the review. In this subset, intervention surrogates had lower scores for decisional conflict measured on the Decisional Conflict Scale (mean difference ‐0.30, 95% CI ‐0.61 to 0.01, reduction of 0.3 to 0.4 units considered meaningful) and were more likely than participants in the control group to discuss feeding options with a clinician (RR 1.57, 95% CI 0.93 to 2.64), but imprecision meant that there was significant uncertainty about both results.
Authors' conclusions
Very little high quality work has been completed exploring palliative care interventions in advanced dementia. There were only two included studies in this review, with variation in the interventions and in the settings that made it impossible to conduct a meta‐analysis of data for any outcome. Thus, we conclude that there is insufficient evidence to assess the effect of palliative care interventions in advanced dementia. The fact that there are six ongoing studies at the time of this review indicates an increased interest in this area by researchers, which is welcome and needed.
Keywords: Aged, Humans, Caregivers, Decision Making, Dementia, Dementia/nursing, Family, Outcome Assessment (Health Care), Palliative Care, Palliative Care/methods, Randomized Controlled Trials as Topic
Palliative care for people with advanced dementia
Review question
In this research, we wanted to see if palliative care helps people with advanced dementia or helps their family or carers. We also wanted to describe how researchers tried to measure the effect of palliative care.
Background
People with advanced dementia have serious memory problems and have problems making simple decisions. They are usually no longer able to communicate by talking. They need a lot of help from their carers. People with advanced dementia can live for a long time. It is very hard to say exactly how long a person with advanced dementia will live.
Palliative care (or end‐of‐life care) is a particular way of caring for people who have diseases that cannot be cured. The main aims of palliative care are to reduce pain and to maintain the best possible quality of life as death approaches. Palliative care is used a lot with people with cancer but is not used much for people with advanced dementia.
Study characteristics
We examined the research published up to January 2016. We found only two suitable studies (189 people), both from the US. We also found six studies that were underway but the results were not yet published.
Key results
One study found that having a small team of doctors and nurses trained in palliative care made little difference to how people with advanced dementia were treated while in hospital. But, having this special team meant that more people had a palliative care plan when they were discharged from hospital. The other study measured if giving written information to relatives explaining the different methods that can be used to feed people with advanced dementia helped either the relatives or the person. This study found that giving relatives this information made it a little easier for relatives to make decisions about what methods would be used to feed the person with dementia.
Certainty of evidence
We only found two studies and the two palliative care methods in these studies were very different. We cannot be very certain about how accurate either of these results reported here are, partly because only a small number of people took part in the studies. So from these studies, it is hard to be sure whether palliative care makes a difference to people with advanced dementia.
Final thoughts
Little research has been done about people with advanced dementia, often because of ethical concerns. However, although it is hard to do research with people with dementia, more well‐designed studies are required to work out how palliative care can be used best in this special population.
Summary of findings
Summary of findings for the main comparison.
Palliative care team in acute hospital
| Palliative care team in acute hospital | ||||||
| Patient or population: people with advanced dementia Setting: acute hospital Intervention: palliative care team Comparison: usual care | ||||||
| Outcomes3 | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with palliative care team | |||||
| Palliative care plan developed during hospitalisation | Study population | RR 5.84 (1.37 to 25.02) | 99 (1 RCT) | ⊕⊝⊝⊝ Very low 1,2 | ‐ | |
| 3.9 per 100 | 22.9 per 100 (5.4 to 98.1) | |||||
| Palliative care plan adopted during an acute hospital admission | Study population | RR 5.31 (0.26 to 107.77) | 99 (1 RCT) | ⊕⊝⊝⊝ Very low 1,2 | ‐ | |
| 0 events in control (no plan adopted in usual care group) so not possible to calculate an absolute effect. | ||||||
| Palliative care plan available on discharge from an acute hospital | Study population | RR 4.50 (1.03 to 19.75) | 99 (1 RCT) | ⊕⊝⊝⊝ Very low 1,2 | ‐ | |
| 3.9 per 100 | 17.6 per 100 (4.0 to 77.5) | |||||
| Decision to forgo CPR in hospital | Study population | Not estimable | 99 (1 RCT) | ⊕⊝⊝⊝ Very low 1,2 | Data on this outcome were reported per admission (not per participant). From these data, there was no evidence that the intervention affected decisions to forgo CPR in hospital. | |
| Data not reported in a way which allowed calculation of risk per participant. | ||||||
| Death in acute hospital | Study population | RR 1.06 (0.53 to 2.13) | 99 (1 RCT) | ⊕⊝⊝⊝ Very low 1,2 | ‐ | |
| 23.5 per 100 | 24.9 per 100 (12.5 to 50.1) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CPR: cardiopulmonary resuscitation; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels for very serious risk of bias (risk of performance bias and contamination of controls high, unclear risk of selection bias).
2 Downgraded two levels for very serious imprecision (single study with few events and wide confidence interval).
3 This 'Summary of findings' table shows only outcomes measured in this comparison.
Summary of findings 2.
Structured decision aid on feeding options
| Structured decision aid on feeding options | ||||||
| Patient or population: people with advanced dementia Setting: nursing homes Intervention: structured decision aid on feeding options Comparison: usual care | ||||||
| Outcomes3 | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with structured decision aid on feeding options | |||||
| Decisional conflict in family/carers assessed with: Decisional Conflict Scale Scale from 0 to 100, lower indicates less decisional conflict Follow‐up: mean 3 months | The mean decisional conflict in family/carers was 1.93 on the Decisional Conflict Scale | The mean decisional conflict in family/carers in the intervention group was 0.3 lower on the Decisional Conflict Scale (0.61 lower to 0.01 higher) | ‐ | 90 (1 RCT) | ⊕⊝⊝⊝ Very low 1,2 | Only a subset of the study population met the review inclusion criteria, so re‐analysis of data from subset required. |
| Discussion on feeding with physician/nurse/physician assistant Follow‐up: mean 3 months | Study population | RR 1.57 (0.93 to 2.64) | 90 (1 RCT) | ⊕⊝⊝⊝ Very low 1,2 | Only a subset of the study population met the review inclusion criteria, so re‐analyse of data from subset required. | |
| 31.8 per 100 | 50.0 per 100 (29.6 to 84.0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level for serious risk of bias (high risk of bias due to lack of blinding of participants and of outcome assessors).
2 Downgraded two levels for very serious imprecision (small sample size and wide confidence intervals).
3 This 'Summary of findings' table shows only outcomes measured in this comparison.
Background
Description of the condition
Dementia is a chronic, progressive and ultimately fatal neurodegenerative disease with several different causes. It is estimated that there were 44.35 million people worldwide living with dementia in 2013 (Prince 2013). This prevalence is projected to double every 20 years to approximately 135.46 million by 2050 (WHO 2012). The incidence of dementia is estimated at 7.7 million new cases per year, or one new case every four seconds and the prevalence doubles with every five‐year increment in age after 65 years. In a 10‐year longitudinal study of 18,248 people aged 65 years and over in the UK, the overall prevalence of dementia at death was 30% and there was a marked increase in such deaths with age: from 6% of people aged 65 to 69 years up to 58% of people aged 95 years and older at time of death (Brayne 2006). Therefore, the provision of appropriate care to the growing number of older people living and dying with dementia is an issue of immense clinical and public health importance.
Although not a normal part of ageing, dementia affects mainly older people, eroding their cognitive and functional abilities and their social skills, often leading to an increase in challenging behaviours and low mood. People with dementia experience a gradual decline in abilities over an extended period, but without abrupt functional or physical health changes that can be used to clearly identify the final, terminal phase of the disease. Advanced or end‐stage dementia is characterised by profound cognitive impairment, inability to communicate verbally, complete functional dependence, and often, dysphagia and double incontinence. People with advanced dementia are at increased risk of infections, for example, urinary tract infections and pneumonia, typically become bed‐ or chair‐ bound, increasing the risk of developing pressure ulcers (Capon 2007).
Advanced dementia is typically defined as having a formal diagnosis of dementia by a clinician, with dementia staged by a validated tool, for example, the Functional Assessment Staging Test (FAST) (Reisberg 1982). Reported six‐month mortality rates for people with advanced dementia of 25% (Mitchell 2009), consistent with high mortality rates among people with advanced dementia from other studies (Morrison 2000a; Mitchell 2004), indicate a life expectancy similar to that in conditions generally recognised as terminal, for example, metastatic breast cancer (Mitchell 2009). Therefore, advanced dementia can be regarded as a terminal condition, where the focus of much, though not necessarily all, of the care provided is palliative, maximising comfort and quality of life, rather than curative. However, studies have shown that people with advanced dementia are often subject to unnecessary investigations during the terminal phase of their illness (Morrison 2000a; Mitchell 2009), and have less analgesia prescribed in the last six months of life compared to people without a cognitive impairment (Morrison 2000b). Failure to recognise and treat pain in dementia is widespread and the risk increases with increased severity of the disease (Scherder 2005). There is also evidence of a high prevalence of antimicrobial treatment in nursing home residents with advanced dementia (Di Giulio 2008; Givens 2010), including evidence that antimicrobial treatment intensifies significantly as people approach death (D'Agata 2008). Thus, usual care of people with advanced dementia is not universally underpinned by a palliative approach.
There are important differences between dementia and other terminal diseases. In dementia, prognosis is less predictable and the trajectory of the disease varies: without a comorbidity, the mean time from diagnosis to death depends strongly on age at diagnosis, varying from 8.3 years for people diagnosed aged 65 to 70 years to 3.4 years for people diagnosed in their 90s (Brookmeyer 2002). Shuster reported that advanced dementia can last two to three years (Shuster 2000), but even for people with advanced dementia, estimating prognosis is still difficult. Medical and nursing home staff overestimate prognosis in advanced dementia (Mitchell 2004), and proposed mortality risk models provide, at best, only modest accuracy in predicting six‐month survival (Mitchell 2009; Mitchell 2012).
One systematic review concluded that there was a need to identify reliable, sensitive and specific prognosticators of mortality in advanced dementia (Brown 2012). Unlike other leading causes of death, advanced dementia is characterised by persistently severe disability during the last year of life (Gill 2010). In addition, the diagnosis and evaluation of pain is more difficult due to challenges communicating with the person with advanced dementia. People with advanced dementia are not always able to express their wishes about their own current and future care, due both to their very limited speech and to their lack of capacity to make decisions (Allen 2003). Thus, this adds to the complexity involved in meeting current care needs and in developing an advance care plan, if a plan is not already in place. Further, clinicians or nurses are not always sensitive to non‐verbal means of communicating pain and distress by people with dementia (Hubbard 2002;Allan 2014).
Palliative care has focused traditionally on care for people with cancer but for more than a decade, there have been increased calls worldwide to extend palliative care services to include all people with life‐limiting illnesses in need of specialist care, including people with dementia (Davies 2004; Australian Government 2006; National Council for Palliative Care 2006; Cahill 2012). In the US, there have been some specialist hospices for people with advanced dementia for some time (Volicer 1994), and there has been a significant increase in the provision of hospice care for people with dementia since the mid‐2000s (Torke 2010; Alzheimer's Association 2014). But appropriate care is still not consistently available across the US for people with advanced dementia (Kim 2005; Mitchell 2007).
Globally, some examples of good practice in palliative care services for people with dementia have emerged but, overall, people with dementia tend to die in residential care, in acute hospitals or at home without palliative interventions (Houttekier 2010; Parker 2011; Ryan 2012). There is some evidence of good palliative care practice for people with dementia in low and middle income countries (Shaji 2009), but palliative care in general is underdeveloped in these regions (Lamas 2012).
The European Association of Palliative Care (EAPC) published a white paper providing a definition, for the first time, of optimal palliative care for people with dementia, based on a Delphi exercise involving experts from 23 countries (van der Steen 2014). Palliative care is defined by the EAPC as "the active, total care of the patients whose disease is not responsive to curative treatment. Control of pain, of other symptoms, and of social, psychological and spiritual problems is paramount. Palliative care is interdisciplinary in its approach and encompasses the patient, the family and the community in its scope. In a sense, palliative care encapsulates the most basic concept of care ‐ that of providing for the needs of the patient wherever he or she is cared for, either at home or in the hospital. Palliative care affirms life and regards dying as a normal process; it neither hastens nor postpones death. It sets out to preserve the best possible quality of life until death" (EAPC 1998).
Description of the intervention
In this review, we included and appraised interventions aimed at improving palliative care delivered to people with advanced dementia. An intervention can impact one or more of the following domains:
the person with dementia, focusing on managing pain or on psychological, social or spiritual dimensions of the person with dementia;
the family/carer, with an emphasis on carer well‐being, carer burden and bereavement support;
the quality of care, which may include interventions such as advance care planning, staff education programmes or the organisation and delivery of care;
the interventions may focus on individual components of care, for example, pain management, or they may be multi‐component interventions aimed at changing the way care is delivered and at improving communication between clinicians, professional carers, the person with dementia and the family.
How the intervention might work
There is some evidence of the benefits of palliative care teams, mainly for people with cancer (Higginson 2003; Gomes 2013), but evidence on the effects of other palliative care interventions is inconclusive (Candy 2012; Chan 2016). Given the complexity of managing people with advanced dementia in the terminal stages of their disease, we anticipated that several different types of interventions could work to improve care in advanced dementia. It is likely that the mechanism by which the interventions may work will also vary significantly, for example:
for the person with advanced dementia: by providing relief from pain, avoiding unnecessary investigations, medications and transitions, and by increasing comfort;
for the family: by increasing their understanding of what to expect during the dying process, by maximising communication with healthcare professionals, by helping families cope with the illness and bereavement, and by reducing the care burden on family carers;
on the organisation of care: by placing the person with advanced dementia at the centre of the care process, by raising the level of awareness of the needs of the person with advanced dementia and by enhancing the communication skills of professional carers.
Why it is important to do this review
There is an increased focus worldwide on extending palliative care to all those in need of it, as evidenced by the 2014 white paper from the EAPC defining optimal palliative care for people with dementia (van der Steen 2014). There is a need to synthesise the evidence available on interventions that improve care for people with advanced dementia for policy makers and clinicians. The chronic disease course of dementia gives families, carers, clinicians and, during the early stages of the disease, the person with dementia, the opportunity to look ahead and plan for the final stages of care. Such decisions should be underpinned by good‐quality evidence.
There is potential for some overlap between this review and the Cochrane Review completed by Hall 2011 entitled Interventions for improving palliative care for older people living in nursing care homes. However, our review differs from Hall 2011 in that it focuses on people with advanced dementia in need of palliative care, living in any setting and includes both interventions that focus on individual components of palliative care, for example, pain management, and multi‐component service interventions.
Objectives
To assess the effect of palliative care interventions in advanced dementia and to report on the range of outcome measures used.
Methods
Criteria for considering studies for this review
Types of studies
Because of the complexity of conducting randomised controlled trials (RCTs) with people with advanced dementia, we anticipated few RCTs. Therefore, we considered it necessary to include a broader range of controlled comparison studies, to help us to determine the effect of interventions to improve care in advanced dementia. Therefore, we considered RCTs, trials where allocation was truly random (e.g. random number table); non‐randomised controlled trials (nRCTs), where allocation was not truly random (e.g. alternation), controlled before‐and‐after studies (CBA) and interrupted time series (ITS) studies for inclusion in this review.
We used the criteria defined in the Cochrane Effective Practice and Organisation of Care (EPOC) Review Group guidelines (EPOC 2013) for inclusion of CBA and ITS studies, as follows:
CBA studies must have had at least two intervention sites and two control sites;
ITS studies must have had a clearly defined point in time when the intervention occurred and at least three data points before and three after the intervention.
Types of participants
Adults of either gender, with dementia of any type staged as advanced by a recognised and validated tool, such as stage 6d or above on the FAST (Reisberg 1988), CDR‐3 (Severe) on the Clinical Dementia Rating (CDR) Scale (Hughes 1982), stage 7 on the Global Deterioration Scale (GDS) (Reisberg 1982), or any other validated measure. We also included studies where the participants were informal or paid carers of people with advanced dementia.
We anticipated that there would be few studies where all participants had advanced dementia. Therefore, we decided a priori to include studies where separate results for people with advanced dementia were available or where more than 80% of the study population had advanced dementia, as defined above. Participants could be living in their own homes or with a family member, in supported housing, in any type of long‐term care facility, in a hospice or in hospital.
Types of interventions
We included clinical and non‐clinical interventions including one or more of the following:
assessment and management of physical, psychological and spiritual symptoms of the person;
advance care planning, including decision‐aid interventions for family carers/surrogates;
management of transition(s) of the person with advanced dementia from one care setting to another;
education and training on living and dying with advanced dementia for family members;
education and training on advanced dementia for clinicians and professional care staff;
changes in the organisation of care to incorporate a palliative approach to care for the person with advanced dementia.
Comparison
We prespecified the following comparisons:
palliative care interventions versus usual care or optimised usual care;
palliative care intervention versus another palliative care intervention.
Types of outcome measures
Primary outcomes
Improvement of care. We anticipated that many different outcomes would have been measured in studies included in this review, using many different measurement scales. Therefore, we reported on all outcomes used in the included studies and aimed to categorise them using the "domains and dimensions of outcome measures in palliative care" (Bausewein 2011). We planned to analyse separately outcomes for the person with advanced dementia, outcomes related to the family or carer, and outcomes related to the quality of care. We looked for outcomes covering both beneficial effects and adverse events. We did not exclude studies on the basis of the outcomes measured.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialized Register. The search terms were: palliative OR "end of life" OR dying.
The Information Specialists of the Cochrane Dementia and Cognitive Improvement Group maintain ALOIS, which contains dementia and cognitive improvement studies identified from:
monthly searches of several major healthcare databases: MEDLINE; Embase; CINAHL; PsycINFO and LILACS;
monthly searches of several trial registers: metaRegister of Controlled Trials; Umin Japan Trial Register and World Health Organization (WHO) portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical Trials Register; German Clinical Trials Register; Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
quarterly search of the Cochrane Library's Central Register of Controlled Trials (CENTRAL);
monthly searches of several grey literature sources: ISI Web of knowledge Conference Proceedings; Index to Theses and Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
In addition, we performed separate searches to ensure we retrieve the most up‐to‐date results. The search strategies run are in Appendix 1.
Data collection and analysis
We developed the methods used in this Cochrane Review in accordance with recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
After merging search results and discarding duplicates, two review authors (EM, SC) independently screened the titles and abstracts of all identified citations to identify potential studies. We classified the citations into three groups: 'exclude', 'potentially relevant' or 'unsure'. We excluded papers classified by both review authors as 'exclude'.
We retrieved the full‐text versions of all 'potentially relevant' and 'unsure' citations for definitive assessment of eligibility. We obtained sufficient translations of non‐English citations to allow us to judge whether to include or exclude the studies. For conference abstracts, we searched for related publications, and, when unable to find any, we contacted the study authors to see whether any further unpublished data were available. Two review authors (EM, SC) independently screened the full texts for a comprehensive assessment against the inclusion criteria. We resolved any disagreements through discussion, and when required, we consulted with all the review team. We used EndNote software to manage citations.
Data extraction and management
We designed and tested a data extraction form. Where possible, we obtained the following information for each included study:
data on the inclusion criteria for the original intervention (study design; setting, including the country; details on the place of residence of participants; types of participants; type of intervention; type of comparator, outcomes measured);
number of participants eligible, number randomised and reasons for not including eligible participants in the study, including both the person with dementia and carers;
length of follow‐up, number of follow‐up points;
participant characteristics, including details on diagnosis, how severity was staged and, where appropriate, details of comorbidity/comorbidities;
carer/family member characteristics, including involvement in delivering care to the person with advanced dementia;
details about the intervention (components, length, mode of delivery, materials given to participants, providers, level of contact with family, etc.), comparison (including definition of usual care);
data to assess the risk of bias of the original trial (randomisation; blinding of participants and personnel; description of dropouts, withdrawals and missing data; details on possible contamination between control and intervention groups; and selective outcome reporting);
baseline and end of intervention data on outcomes of interest for the review, scales used to measure outcomes.
Two review authors (EM, SC) extracted data using the agreed form and resolved discrepancies through discussion. We had planned to consult with another review author if required to reach agreement but this was not necessary. When information regarding any data were missing, unclear or incomplete, we attempted to contact authors of the original reports to request further details. We entered data in duplicate into Review Manager 5 (RevMan 2014), and checked for accuracy. When information regarding any data was missing, unclear or incomplete, we attempted to contact authors of the original reports to request further details.
Assessment of risk of bias in included studies
Two review authors (EM and DD) independently assessed risk of bias for each included study. We used the Cochrane tool for assessing risk of bias (Table 8.4.a, Higgins 2011). We had planned to use additional guidance from the Cochrane EPOC group for CBAs and ITS studies (EPOC 2013), but this was not necessary as both included studies were RCTs. We resolved any disagreements by discussion and did not need to consult a third review author. The risk of bias assessors knew the identity of the publication and the author information for each study. We attempted to contact study authors for clarification where the methodology was not clearly described in the study report.
We considered selection bias and reporting bias across each study. We planned to report performance bias, detection bias and attrition bias for each outcome (e.g. mortality) or class of outcomes (e.g. subjective outcomes). However, because all outcomes reported in this review required a degree of subjective assessment, we reported risk of bias only by group (class of outcome).
Selection bias: random sequence generation
We assessed the risk that the random sequence generation method used did not produce comparable groups, scoring selection bias thus:
for RCTs, if the sequence generation process was clearly random (e.g. use of random number table): low risk;
for RCTs, if the sequence generation process was not specified in the paper and not available from the authors: unclear risk;
for nRCTs, CBA studies and ITS studies: high risk (but both included studies were RCTs).
Selection bias: allocation concealment
We assessed the risk that the intervention allocation could have been foreseen (was not concealed adequately) in advance of or during recruitment or could have been changed after assignment of participants to intervention groups. We scored selection bias thus:
if sealed opaque envelopes were used, if randomisation and allocation was performed on all participants or units at the same time after recruitment was completed, if a person outside the study team was responsible for revealing the allocation or if some central allocation process was used (e.g. central telephone contact): low risk;
any inadequate concealment of allocation (e.g. allocation list available to researchers before recruitment of some participants): high risk;
if the allocation concealment process was not specified in the paper and not available from the authors: unclear risk;
for nRCTs, CBA studies and ITS studies: high risk (but both included studies were RCTs).
Performance bias: blinding participants and personnel
Given the nature of many palliative interventions, it is not possible to blind participants and study personnel to the interventions. However, we described the methods used, if any, to blind participants, including family members, and study personnel to the intervention and scored selection bias thus:
for all outcomes, if participants and study personnel were blinded or if we judged that the lack of blinding was unlikely to impact results: low risk;
when we considered lack of blinding of participants and study personnel was likely to impact a given outcome: high risk;
when it was not clear whether lack of blinding of participants and study personnel impacted a particular outcome: unclear risk.
Detection bias: blinding of outcome assessors
We attempted to ascertain whether outcome assessors were blinded to the intervention and scored detection bias thus:
for all outcomes assessed blindly: low risk;
for objective outcomes (e.g. mortality), where outcome assessors were not blinded: low risk;
for subjective outcomes (e.g. pain), where outcome assessors were not blinded: high risk;
if it was not clear whether outcome assessors were blinded for an outcome that we considered would be impacted by lack of blinding: unclear risk.
Attrition bias: incomplete outcome data
We explored whether dropouts and withdrawals, and reasons why they occurred, were reported, with a particular focus on establishing if missing data were balanced across groups and we scored attrition bias thus:
if less than 20% of data were missing, and missing data were balanced across groups: low risk;
if either more than 20% of data were missing or missing data were not balanced across groups: high risk;
if the percentage of missing data were not clear or it was unclear whether the missing data were equally divided across groups: unclear risk.
Reporting bias
We compared the outcomes reported in the Results section of the study publications with the outcomes listed in the Methods section of the paper reporting the findings and the study protocol (where available) to identify any selective outcome reporting and scored the risk of reporting bias thus:
if it was clear that all prespecified outcomes and all key expected outcomes were reported: low risk;
if all the study's prespecified outcomes were not reported or if one or more of the reported primary outcomes were not prespecified: high risk;
if outcomes of interest were not reported completely or if a key outcome that one would expect to have been reported was not reported: high risk;
if there was doubt whether the outcomes reported included all outcomes measured: unclear risk.
Other potential sources of bias
We examined the study reports for other potential sources of bias (e.g. the risk of contamination of controls), and scored the risk of bias from other sources thus:
study appeared to be free of other sources of bias: low risk;
there was at least one other important risk of bias (e.g. extreme baseline imbalance not adjusted for in analysis or contamination of controls): high risk;
if there was insufficient information to assess whether another important source of bias existed or if there was not sufficient evidence that an identified problem would introduce bias: unclear risk.
For cluster RCTs, we assessed these additional sources of bias (Section 16.3.2, Cochrane Handbook for Systematic Reviews of Interventions, Higgins 2011):
recruitment bias (e.g. were people recruited after clusters were randomised or were inclusion/exclusion criteria applied differently in different arms?);
baseline imbalance between the clusters;
incorrect analysis ‐ was there evidence of adjustment in analysis for cluster effect?
For each of these sources, we rated the risk of bias as follows:
if there was clear evidence that no risk of bias was introduced: low risk;
if there was evidence of a problem and no adjustment had been made in the analysis to counteract it: high risk;
if insufficient information was available to make a decision on the risk of bias from a specific source: unclear risk.
Summary of risk of bias
All outcomes reported in the 'Summary of findings' table required a degree of subjective assessment. Therefore, we considered that all outcomes were subjective and assessed the overall risk of bias for all outcomes as a group, as follows (guided by Table 8.7.a in Higgins 2011):
if most information was from studies at low risk of bias: low risk;
if the proportion of information from studies at high risk of bias was sufficient to affect the interpretation of the results: high risk;
if most information was from studies at low or unclear risk of bias: unclear risk.
At an individual study level, we rated studies as high quality when they were at low risk of bias for allocation concealment and incomplete outcome data. Finally, we incorporated the results of the risk of bias assessment into the review through systematic narrative description and commentary. We did not conduct a meta‐analysis, therefore, were unable to explore the effect of the risk of bias through a sensitivity analysis based on trial quality, as planned.
Additional detail on methods of analysis for 'Risk of bias' assessment that could be used in future updates of this review, should a meta‐analysis of data be possible, are available in the protocol for this review (Murphy 2015).
Measures of treatment effect
For dichotomous data, we planned to present results as summary risk ratios (RR) with 95% confidence intervals (CIs). For continuous data, we planned to use the mean difference (MD) with 95% CIs where outcomes were measured using the same scale or in the same way in the included studies. We planned to use change‐from‐baseline data, or, if these were not available, final value scores. We planned to use the standardised mean difference (SMD) with 95% CIs if studies measured the same outcomes but use different measurement scales (Higgins 2011).
Additional details on methods to address unit of analysis issues, assessment of heterogeneity, assessment of reporting bias, data synthesis, and subgroup and sensitivity analysis that could be used in updates of this review, should a meta‐analysis of data be possible, are available in the protocol for this review (Murphy 2015).
Dealing with missing data
Where data were missing from published reports, we contacted study authors to request data for included studies. For included studies, we noted the level of attrition, per group, and per outcome or group of outcomes.
Data synthesis
We planned to use Review Manager (RevMan) to conduct statistical analysis; however, the included studies were too disparate in terms of interventions, settings and outcomes to allow pooling of results in a meta‐analysis, so we described the results of the trials using a narrative summary.
Summarising and interpreting results
We used the GRADE system to assess the certainty of evidence behind each outcome, taking account of risk of bias in the contributing studies, imprecision of the effect estimate, inconsistency between studies, indirectness of the evidence and possible publication bias (Guyatt 2008). For each comparison, we constructed a 'Summary of findings' table that was an adaptation of that produced from the GRADE Development Tool software (GRADEpro 2014). To identify the seven most important outcomes for inclusion in the 'Summary of findings' table, we conducted a priority‐setting exercise. An online survey listed all the outcomes measured in the included studies and each author on the review team independently ranked the five outcomes (out of the 31 outcomes measured in the included studies) they considered the most important. From this process, we identified the top seven outcomes for inclusion in the 'Summary of findings' table as:
palliative care plan adopted during an acute hospital admission;
palliative care plan available on discharge from an acute hospital;
decisional conflict in family/informal carers;
discussions on feeding with a physician/nurse/physician assistant;
palliative care plan developed during hospitalisation;
decision to forgo cardiopulmonary resuscitation (CPR) in hospital;
death in an acute hospital.
Results
Description of studies
See the Characteristics of included studies table, Characteristics of excluded studies table and the Characteristics of ongoing studies table.
Results of the search
We identified 1648 citations with potential for inclusion from our initial electronic search in January 2015 and five citations from other sources. A second search in February 2016 identified a further 157 citations (see Figure 1). After removing duplicates, we screened the titles and abstracts of 1535 citations and excluded 1457 citations. We reviewed the full text of the remaining 78 citations for a more detailed evaluation. We contacted authors of 13 studies to clarify methodological queries, 11 authors responded, one of whom re‐analysed data for the purposes of this review (Hanson 2011). Of the full‐text studies reviewed, two studies, each reported in two citations, met our inclusion criteria and were included in the review: one individually randomised controlled trial (Ahronheim 2000) and one cluster RCT (Hanson 2011). We identified no completed quasi‐randomised studies, CBAs or ITS.
Figure 1.
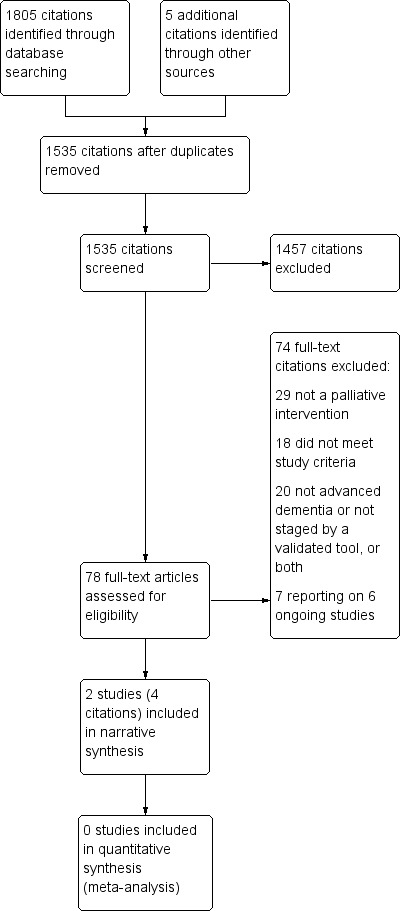
Study flow diagram.
Included studies
The individually randomised controlled trial involved 99 participants with advanced dementia (Ahronheim 2000) and the cluster RCT included 256 dyads, where each person with advanced dementia was recruited with a surrogate decision‐maker; 90 dyads in the study population met the advanced dementia criteria of this review (Hanson 2011).
Participants and settings
The Ahronheim study included people with advanced dementia, staged as FAST 6d‐7f, hospitalised for an acute illness (Ahronheim 2000). It was conducted at one acute hospital in the US. The Hanson study included nursing home residents with advanced dementia, staged as GDS 6 or 7, and feeding problems; each resident was enrolled with a surrogate decision maker, defined as the resident's guardian, Health Care Power of Attorney, or the primary family contact and most likely to be involved in clinical decision making (Hanson 2011). Of the 256 dyads in the study, 90 residents were staged as GDS 7, thus meeting the criteria for inclusion in this systematic review, and the authors re‐analysed the data for this subset of participants for the purposes of this review. The study was conducted in 24 nursing homes in the US.
Characteristics of the interventions
The Ahronheim study measured the effectiveness of a palliative care team established at the acute hospital, consisting of a clinical nurse specialist and one or more attending geriatrician(s), who also held academic appointments (Ahronheim 2000). The palliative care team visited each person and discussed their management with the primary healthcare team in the hospital on a daily basis during admission, making recommendations with the goal of enhancing each person's comfort. The palliative care team also discussed participant care with surrogates when possible, in person or by telephone. The control group were treated by the primary care team without the input of the palliative care team.
The Hanson study tested whether a decision aid for surrogates of nursing home residents with advanced dementia improved the quality of decision‐making about feeding options (Hanson 2011). Surrogates received a structured decision aid providing information about dementia; feeding options, including feeding for comfort near the end of life; and the outcomes, advantages and disadvantages of feeding tubes and assisted oral feeding. The decision aid also discussed the surrogate's role in decision making and the surrogates were encouraged to discuss the decision aid with healthcare providers. Control surrogates received usual care, including any information typically provided by healthcare providers.
Outcome measures
In the Ahronheim study, outcomes reported included the total and the mean number of admissions, the number of deaths in hospital, the existence of a palliative care plan, with a breakdown on the number of decisions recorded to forgo each of seven life‐sustaining treatments, details on the use of nine life‐sustaining interventions during admissions and details on the use of four procedures (feeding tubes, mechanical ventilation, tracheostomy and CPR) (Ahronheim 2000). The outcomes measured in this study focused on the process of care, rather than on the outcomes for the participant or the family/carer.
The primary outcome in the Hanson study was decisional conflict of the surrogate at three months (Hanson 2011), measured using the validated Decisional Conflict Scale (O'Connor 1995). Secondary outcomes included knowledge about dementia and feeding options, frequency of feeding discussions between surrogate and care providers and the use of assisted feeding treatments. The primary outcome in this study focused on the family/carer, the secondary outcomes focused mainly on the process of care.
In total, the two studies measured 31 different outcomes and no outcome was measured in both studies.
Excluded studies
We excluded 74 citations at full‐text stage: 29 did not describe a palliative intervention, 18 did not meet study design criteria, 19 either did not include participants with advanced dementia or less than 80% of the participants had advanced dementia and results were not available separately for those with advanced dementia, and one study included participants with dementia but the severity of dementia was not staged by a validated functional scale. The Characteristics of excluded studies table lists details of studies excluded at full‐text stage.
In addition, there were seven citations reporting on six ongoing studies meeting the inclusion criteria for this review; data collection was complete in four studies with analysis underway but results not yet available when this review was drafted (Agar 2015; Boogaard 2013;Einterz 2014; Arcand 2015), and data collection was ongoing in the other two studies (NCT01774799; NCT02211287).
Risk of bias in included studies
For risk of bias assessment (see Characteristics of included studies table), we grouped all outcomes reported in each included study into one group, subjective outcomes, as the measurement of all outcomes was open to some subjectivity. In the Ahronheim study, we judged risk of performance bias as high, and we judged the risk of selection bias as unclear. We also considered there was a high risk of contamination bias as intervention and control participants were managed by the same medical team. We judged the risk of detection, attrition and reporting bias as low (Ahronheim 2000). In the Hanson study, we judged the risk of recruitment bias, performance bias and detection bias as high. We judged the risk of selection bias, reporting bias, attrition bias and other biases as low (Hanson 2011). Figure 2 summarises the risk of bias assessment for both studies.
Figure 2.
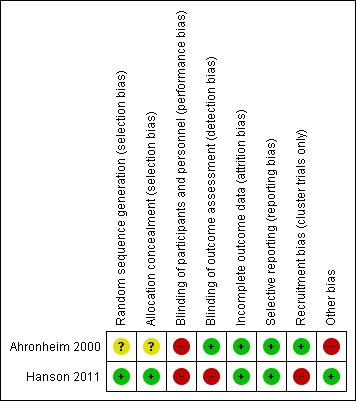
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
We did not pool data due to the heterogeneity between the two trials in terms of the interventions, the outcomes and the times at which the outcomes were measured. For the same reasons, we did not perform subgroup or sensitivity analyses. Instead, the results of the two trials are presented separately.
Ahronheim 2000 provided data on the impact of a palliative care team in an acute hospital. Because the data were from a single study with a small sample size, event rates were low for some outcomes, and there was a high risk of performance bias and contamination bias, we judged the certainty of evidence behind all effect estimates as very low (downgraded due to very serious risk of bias for all outcomes, and downgraded for either or both of inconsistency and imprecision, depending on the outcome).
Drawing on data from one study only (Ahronheim 2000), there was evidence that participants allocated to a palliative care team were more likely to have a palliative care plan developed during hospitalisation than participants in the control group (RR 5.84, 95% CI 1.37 to 25.02; 1 trial, 99 participants; Analysis 1.1). Only two participants, both in the intervention group, had a palliative care plan used during hospitalisation, hence we could not draw conclusions about the effect of the intervention on this outcome. The palliative care plan was usually not available until discharge and availability on discharge was more likely in the intervention group (RR 4.50, 95% CI 1.03 to 19.75; 1 trial, 99 participants; Analysis 1.1).
Analysis 1.1.
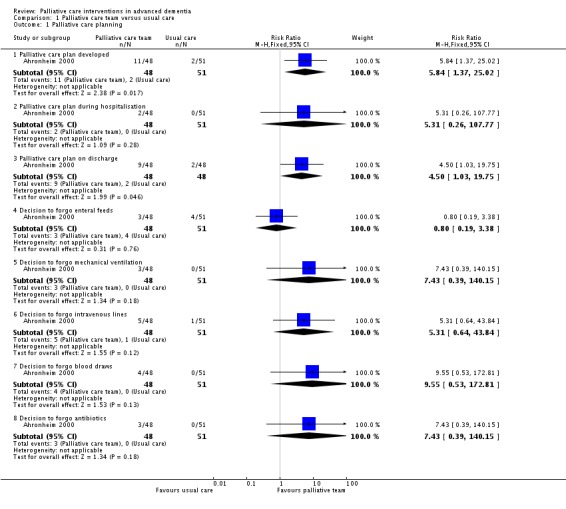
Comparison 1 Palliative care team versus usual care, Outcome 1 Palliative care planning.
There was no difference in mortality in hospital between participants allocated to the palliative care team and participants receiving usual care (RR 1.06, 95% CI 0.53 to 2.13; 1 trial, 99 participants; Analysis 1.2). There was no difference in the mean number of hospital admissions per participant between the groups (MD 0.04, 95% CI ‐0.74 to 0.82; Analysis 1.3).
Analysis 1.2.
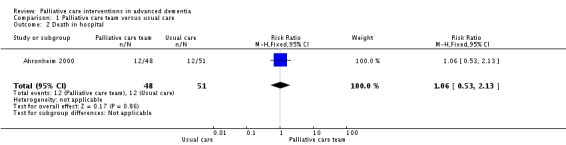
Comparison 1 Palliative care team versus usual care, Outcome 2 Death in hospital.
Analysis 1.3.
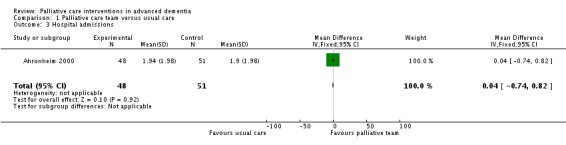
Comparison 1 Palliative care team versus usual care, Outcome 3 Hospital admissions.
Data reported suggest that the intervention had no impact on decisions to forgo CPR in hospital or on discharge. Only a small number of decisions were made to forgo treatments other than CPR. There was little use of mechanical ventilation, tracheostomy or CPR in either group. The study reported that participants in the intervention group were less likely to have intravenous therapy during admission, but there was no impact on the use of other life‐sustaining treatments, including systemic antibiotics, daily phlebotomy or the number of new feeding tubes inserted. However, data on decisions to forgo CPR and the use of life‐sustaining treatments are reported based on the number of admissions rather than the number of participants, thus the effect sizes are more precise than they should be and therefore are not reported here.
One study reported the effect of a decision aid for surrogate decision‐makers of nursing home residents with advanced dementia (Hanson 2011). For the purposes of this review, the authors re‐analysed the data for 90 study dyads with advanced dementia staged as GDS 7 (35% of total study population), the participant inclusion criterion for the review. Because the data were drawn from a reanalysis of a small subgroup from a single study and the risk of recruitment, performance and detection biases were high, we judged the certainty of the evidence for all outcomes as very low (downgraded due to very serious risk of bias and serious inconsistency).
In this subset, the intervention surrogate decision‐makers had lower total scores on the Decisional Conflict Scale (MD ‐0.30, 95% CI ‐0.61 to 0.01; 1 trial, 90 participants; Analysis 2.1) and were more likely than controls to discuss feeding options with a physician, nurse practitioner or physician assistant (RR 1.57, 95% CI 0.93 to 2.64; Analysis 2.2), although in both outcomes the CIs included no difference. A change of 0.3 to 0.4 on the Decisional Conflict Scale is considered a meaningful change (O'Connor 1993).
Analysis 2.1.
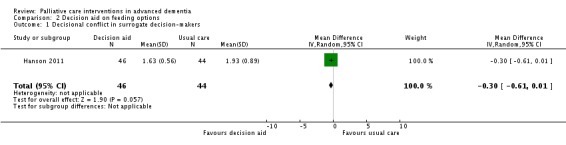
Comparison 2 Decision aid on feeding options, Outcome 1 Decisional conflict in surrogate decision‐makers.
Analysis 2.2.
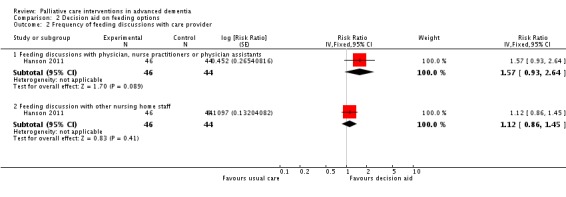
Comparison 2 Decision aid on feeding options, Outcome 2 Frequency of feeding discussions with care provider.
Participants whose surrogate decision‐makers received the decision aid intervention were more likely to receive a modified diet (RR 1.19, 95% CI 0.31 to 4.54; 1 trial, 90 participants; Analysis 2.3) and were more likely to be on a specialised dysphagia diet (RR 1.30, 95% CI 1.09 to 1.56; Analysis 2.3). The intervention had no impact on the use of other assisted oral feeding techniques. No surrogates in either group had made an explicit choice for or against a feeding tube at three months.
Analysis 2.3.
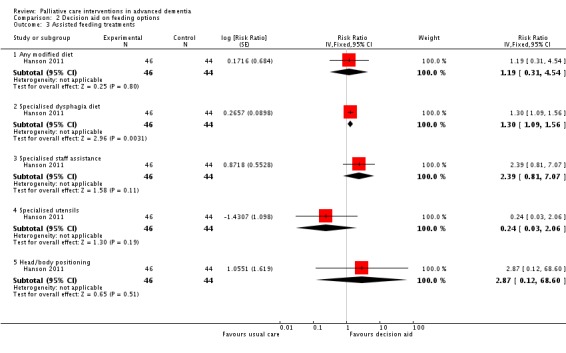
Comparison 2 Decision aid on feeding options, Outcome 3 Assisted feeding treatments.
Discussion
Summary of main results
The primary aim of this review was to assess the effect of palliative care interventions in advanced dementia. We included two trials. Although the populations in the two included studies were similar, differences between the interventions and the outcomes measured meant that it was not possible to conduct a meta‐analysis, that is, to pool data meaningfully across the two studies. One study assessed the effect of a palliative care team in an acute hospital (Ahronheim 2000), the other study assessed the impact of a decision aid on the quality of decision‐making about feeding options by surrogate decision‐makers of nursing home residents with advanced dementia (Hanson 2011). The Hanson 2011 study required a reanalysis of the study data to include only data pertaining to a subset of the study population that met the inclusion criteria of this systematic review.
While one study reported that with a palliative care team intervention in acute hospitals, participants in the intervention group were more likely to have a palliative care plan, the numbers were small and the palliative care plan was typically not in place until discharge (Ahronheim 2000). It was also less likely that intravenous therapy was used in participants in the intervention group during hospitalisation but the intervention did not impact the use of any other life‐sustaining treatments or procedures.
In the second study, data for the subset of participants meeting the review definition of advanced dementia was reanalysed (Hanson 2011). From the reanalysis of this subset, we found some evidence that a decision aid helps to reduce decisional conflict in surrogates and leads to increased discussions between surrogates and healthcare providers on feeding options, but there was significant uncertainty about both of these results. However, the original study, drawing on data from the full study population, provided stronger evidence that the intervention has a positive impact for both of these outcomes, which gives us more confidence that there is a true effect. The decision aid also resulted in more residents with advanced dementia being on a specialised dysphagia diet.
Overall completeness and applicability of evidence
The number of included studies, the variation in the interventions and settings of the two included studies and the fact that it was not possible to conduct a meta‐analysis of data for any outcome provided insufficient evidence to assess the effect of palliative care interventions in advanced dementia. The two included trials give an indication of the range of outcomes that have been measured to date but it is not possible to have a meaningful discussion on outcomes based on only two trials.
The definition of advanced dementia for this review led to the exclusion of some quality studies conducted on a more general population of people with dementia, but our definition does retain a focus on the most vulnerable people with dementia. The latter are a particularly important group given their proximity to death and their need for palliative interventions across a range of activities. They are also a vulnerable group given the difficulty of communication that occurs with advanced dementia and of being understood by care providers even when non‐verbal communication is attempted. The existing lack of data on optimal palliative care interventions to meet the needs of this special population highlights the importance of research focused on this population, despite the challenges.
The fact that there are six ongoing studies at the time of this review indicates an increased interest in this area by researchers and possibly a recognition by ethics committees of the importance of conducting research in vulnerable populations. There are also signs of a growing convergence in the outcomes that are being measured, which means that the results will be more comparable, with potential to conduct a meta‐analysis on some outcomes.
We had planned to use the Bausewein model domains and dimensions of outcome measures in palliative care to report on the outcomes measured in the included studies and to analyse separately outcomes for the person with advanced dementia, outcomes related to family or carers and outcomes related to quality of care (Bausewein 2011). However, due to the low number of studies included and the diversity in outcomes measured, this was not possible. It is worth noting that one study focused entirely on process of care outcomes (Ahronheim 2000), while the primary outcome in the Hanson 2011 study related to the family and the secondary outcomes in this study focused mainly on process of care outcomes. However, the six ongoing studies suggest that there is a growing focus on outcomes of relevance to the person with advanced dementia, for example, comfort in dying and comfort at end of life, and of relevance to the family/carer, for example, satisfaction with care, measured using the End‐of‐Life‐in Dementia (EOLD) suite of measurement tools (Volicer 2001).
Quality of the evidence
Very little high quality work has been completed exploring palliative care interventions in advanced dementia. This review included only two trials, with 189 people with advanced dementia. A meta‐analysis of the data was not possible, due to variations in the interventions, the settings and the outcomes measured. One study had a small sample size and the number of events was very small. The second study required a reanalysis of a subset of the participants in the original study to include only those participants who met the inclusion criteria for this review, resulting in a small sample size that was no longer powered to measure the primary outcome. We downgraded the evidence from both studies by two due to risk of bias and downgraded the evidence because of other factors. Therefore, the certainty of evidence reported in this review is very low.
Potential biases in the review process
This review was conducted as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); therefore, the introduction of bias during the review process was minimised. We are confident that the search strategy identified all relevant studies. Some bias may have been introduced by limiting the reanalysis of data in the Hanson 2011 study to a subset of the original study outcomes but the reanalysis was conducted long after study end specifically for this review.
Limitations of this review
The limitations of this work are related to the small number of studies that met the criteria for inclusion in the review. Quite simply, there has been a dearth of work on palliative care interventions for people with advanced dementia. There are many reasons for so few studies, but an important consideration is the relatively low priority given to palliative care for people with advanced dementia, making it difficult to even imagine different forms of interventions, let alone implement and evaluate them. Methodological issues in respect of randomisation and outcome measures may have also inhibited work in this important area. This may now be changing, given the potential publication of six new studies in this area in the near future, making it possible to conduct more complete reviews, including meta‐analyses.
Authors' conclusions
Ahronheim 2000 advised targeting people with advanced dementia before transfer to acute hospital to allow for discussion on goals of care in a less urgent environment. However, there is a growing consensus internationally that palliative care should be provided across the full continuum of care and across a range of conditions (Davies 2004;UK End of Life Care Strategy 2008;Cahill 2012;WHO 2012), and people with advanced dementia are still dying in acute hospitals in many countries (Houttekier 2010). Therefore, we should not abandon efforts to introduce a palliative care approach with these people when they are admitted to acute hospitals, if an advance care plan is not already in place.
However, very little high quality work has been completed exploring palliative care interventions in advanced dementia. We found only two included studies for this review, with variation in the interventions and in the settings that made it impossible to conduct a meta‐analysis of data for any outcome. Thus, we conclude that there is insufficient evidence to assess the effect of palliative care interventions in advanced dementia. The fact that there are six ongoing studies at time of this review indicates an increased interest in this area by researchers, which is welcome and needed.
The results of the Hanson study suggest that it is worthwhile to further investigate the use of the decision aids among surrogates of people with advanced dementia (Hanson 2011). It is also evident that high‐quality studies of many different palliative care interventions in all settings are required to improve palliative care delivered to people with advanced dementia. The Ahronheim study reminds us of the need to include the acute hospital setting (Ahronheim 2000). Because insufficient evidence is currently available, research is required to identify what the nature of these interventions should be.
Palliative care researchers face many challenges, including the vulnerability of the population from which study participants are recruited; the difficulty in assessing the risks and benefits of participating in the research; and issues around consent, emotional distress and randomisation (Krouse 2004). These challenges are exacerbated when the focus is on people with advanced dementia, particularly related to communication, capacity and appropriate outcome measures. Therefore, there is a need to conduct methodological research to develop best practice guidelines for research in this area.
There is also a clear need for the development of a core outcome set for palliative care for people with advanced dementia. Developing a core outcome set will need to take account of the personhood of people with dementia, including holistic measures that incorporate standard measures such as pain and quality of life alongside functioning and capabilities assessment. This will, in turn, require increased collaboration and interdisciplinary work, bringing together not just clinicians from psychiatry, geriatrics and palliative care, but also expertise in pain management, communication (verbal and non‐verbal), psychology, social gerontology, health economics and even philosophy.
Acknowledgements
We acknowledge Professor Laura Hanson and her team who have kindly reanalysed study data to include a subset of their study population for the purposes of this review. We acknowledge numerous authors we contacted who provided clarification on the methodology of their studies. We would also like to thank Anna Noel‐Storr for conducting the electronic search and helping to screen the citations identified by the second search and Sue Marcus, editor of the Cochrane Dementia and Cognitive Improvement Group for her support. The lead author was supported by a Cochrane Fellowship funded by the Health Research Board, Ireland.
Appendices
Appendix 1. Search strategies and hits retrieved
| Source | Search strategy |
Hits retrieved 30 January 2015 |
Hits retrieved 4 February 2016 |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | palliative OR terminal OR hospice OR dying OR "end of life" | 16 | 0 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946 to present (OvidSP) | 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. exp Palliative Care/ 22. "Hospice and Palliative Care Nursing"/ 23. Terminal Care/ 24. "end of life".ti,ab. 25. palliative.ti,ab. 26. (dying adj3 (care or comfort or relief or strateg* or plan or intervention or pain)).ti,ab. 27. "symptom control".ti,ab. 28. (bereavement adj2 support).ti,ab. 29. or/21‐28 30. 20 and 29 31. randomized controlled trial.pt. 32. controlled clinical trial.pt. 33. random$.ti,ab. 34. groups.ab. 35. drug therapy.fs. 36. placebo.ab. 37. rct.ti,ab. 38. or/31‐37 39. 30 and 38 |
494 | 48 |
| 3. Embase 1974 to 1 February 2016 (OvidSP) |
1. exp *dementia/ 2. dement*.ti,ab. 3. alzheimer*.ti,ab. 4. (lewy* adj2 bod*).ti,ab. 5. (frontotemporal* or FTD or FTLD).ti,ab. 6. or/1‐5 7. exp palliative nursing/ or exp palliative therapy/ 8. hospice care/ or hospice/ or hospice nursing/ or hospice patient/ 9. terminal care/ 10. death/ or dying/ 11. palliative.ti,ab. 12. hospice*.ti,ab. 13. terminal.ti,ab. 14. "end of life".ti,ab. 15. (dying adj3 (care or comfort or relief or strateg* or plan or intervention or pain)).ti,ab. 16. ("symptom control" and (dying or death)).ti,ab. 17. (bereavement adj2 support).ti,ab. 18. or/7‐17 19. 6 and 18 20. randomized controlled trial/ 21. controlled clinical trial/ 22. (randomly adj3 (divide* or shared or allocat*)).ti,ab. 23. placebo.ab. 24. "double‐blind*".ti,ab. 25. "single blind*".ti,ab. 26. RCT.ti,ab. 27. (randomized or randomised).ti. 28. or/20‐27 29. 19 and 28 |
276 | 37 |
| 4. PsycINFO 1806 to January 2016 week 4 (OvidSP) |
1. dement*.ti,ab. 2. alzheimer*.ti,ab. 3. exp Dementia/ 4. (lewy* adj2 bod*).ti,ab. 5. (frontotemporal* or FTD or FTLD).ti,ab. 6. or/1‐5 7. exp Hospice/ or exp "Death and Dying"/ or exp Palliative Care/ or exp Terminally Ill Patients/ 8. hospice*.ti,ab. 9. terminal*.ti,ab. 10. "end of life".ti,ab. 11. (dying adj3 (care or comfort or relief or strateg* or plan or intervention or pain)).ti,ab. 12. ("symptom control" and (dying or death)).ti,ab. 13. (bereavement adj2 support).ti,ab. 14. palliative.ti,ab. 15. or/7‐14 16. 6 and 15 17. exp Intervention/ or exp Clinical Trials/ 18. placebo.ab. 19. randomly.ab. 20. (randomised or randomized or RCT or trial).ti,ab. 21. "double‐blind*".ti,ab. 22. "single blind*".ti,ab. 23. or/17‐22 24. 16 and 23 |
276 | 15 |
| 5. CINAHL (EBSCOhost) 1980 to 31 January 2016 | S1 (MH “Dementia”) S2 TX dement* S3 TX alzheimer* S4 TX “lew* bod*” S5 TX FTLD OR FTD OR frontotemporal S6 S1 OR S2 OR S3 OR S4 OR S5 S7 (MH "Palliative Care") OR (MH "Hospice and Palliative Nursing") OR (MH "Terminal Care") OR (MH "Hospice Care”) S8 TX "end of life” S9 TX palliative OR terminal* OR hospice* OR bereavement S10 S7 OR S8 OR S9 S11 S6 AND S10 S12 (MH "Randomized Controlled Trials”) S13 TX randomised S14 TX randomized S15 AB placebo S16 AB randomly S17 AB "double blind*” S18 AB "single blind*” S19 AB RCT S20 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 S21 S11 AND S20 |
75 | 16 |
| 6. ISI Web of Science ‐ all databases [includes: Web of Science (1945 to present); BIOSIS Previews (1926 to present); MEDLINE (1950 to present); Journal Citation Reports] | TOPIC: (dement* OR alzheimer* OR "lew* bod*" OR frontotemporal OR FTD OR FTLD OR "severe* cognit* impair*") AND TOPIC: (palliative* OR terminal* OR hospice* OR dying OR "end of life" OR bereavement) AND TOPIC: (RCT OR "randomly alloca*" OR randomised OR randomized OR placebo OR "double blind*" OR "single blind*") Timespan: All years. Search language=Auto |
463 | 37 |
| 7. LILACS (BIREME) | demência OR dementia OR demencia OR alzheimer$ [Words] and paliativos OR palliative OR hospice OR terminal OR terminalidade OR morrer OR dying OR morte [Words] and randomizado OR randomised OR randomized OR placebo OR randomly [Words] | 1 | 1 |
| 8. CENTRAL (the Cochrane Library) 2016, Issue 1 | #1 dement* #2 alzheimer* #3 MeSH descriptor: [Dementia] explode all trees #4 "lew* bod*" or DLB or LBD #5 frontotemporal* or FTD or FTLD #6 #1 or #2 or #3 or #4 or #5 #7 palliative #8 terminal* #9 hospice* #10 "end of life" #11 dying #12 bereavement #13 MeSH descriptor: [Palliative Care] explode all trees #14 MeSH descriptor: [Hospice and Palliative Care Nursing] explode all trees #15 MeSH descriptor: [Terminal Care] explode all trees #16 MeSH descriptor: [Palliative Medicine] explode all trees #17 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 #17 and #6 |
131 | 25 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | [condition] dementia OR alzheimer OR alzheimers AND [search terms] palliative OR terminal OR hospice OR dying OR "end of life” Study type: interventional Dates: ALL |
66 | 8 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry ‐ India; Clinical Research Information Service ‐ Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] | [condition] dementia OR alzheimer OR alzheimers AND [intervention] palliative OR terminal OR hospice OR dying OR "end of life” Recruitment status: ALL Dates: ALL |
6 | 0 |
| TOTAL before de‐duplication | 1648 | 187 | |
| TOTAL after de‐duplication | 1382 | 157 | |
Data and analyses
Comparison 1.
Palliative care team versus usual care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Palliative care planning | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Palliative care plan developed | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.84 [1.37, 25.02] |
| 1.2 Palliative care plan during hospitalisation | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.26, 107.77] |
| 1.3 Palliative care plan on discharge | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [1.03, 19.75] |
| 1.4 Decision to forgo enteral feeds | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.19, 3.38] |
| 1.5 Decision to forgo mechanical ventilation | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.43 [0.39, 140.15] |
| 1.6 Decision to forgo intravenous lines | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.64, 43.84] |
| 1.7 Decision to forgo blood draws | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.55 [0.53, 172.81] |
| 1.8 Decision to forgo antibiotics | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.43 [0.39, 140.15] |
| 2 Death in hospital | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.53, 2.13] |
| 3 Hospital admissions | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.74, 0.82] |
| 4 Use of procedures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 New feeding tube | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.65] |
| 4.2 Total feeding tube use | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 4.3 Mechanical ventilation | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.10, 2.77] |
| 4.4 Tracheostomy | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.48] |
| 4.5 Cardiopulmonary resuscitation | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.86] |
Analysis 1.4.
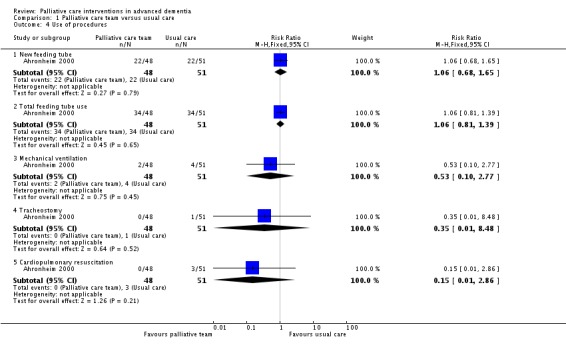
Comparison 1 Palliative care team versus usual care, Outcome 4 Use of procedures.
Comparison 2.
Decision aid on feeding options
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Decisional conflict in surrogate decision‐makers | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.61, 0.01] |
| 2 Frequency of feeding discussions with care provider | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Feeding discussions with physician, nurse practitioners or physician assistants | 1 | 90 | Risk Ratio (Fixed, 95% CI) | 1.57 [0.93, 2.64] |
| 2.2 Feeding discussion with other nursing home staff | 1 | 90 | Risk Ratio (Fixed, 95% CI) | 1.12 [0.86, 1.45] |
| 3 Assisted feeding treatments | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Any modified diet | 1 | 90 | Risk Ratio (Fixed, 95% CI) | 1.19 [0.31, 4.54] |
| 3.2 Specialised dysphagia diet | 1 | 90 | Risk Ratio (Fixed, 95% CI) | 1.30 [1.09, 1.56] |
| 3.3 Specialised staff assistance | 1 | 90 | Risk Ratio (Fixed, 95% CI) | 2.39 [0.81, 7.07] |
| 3.4 Specialised utensils | 1 | 90 | Risk Ratio (Fixed, 95% CI) | 0.24 [0.03, 2.06] |
| 3.5 Head/body positioning | 1 | 90 | Risk Ratio (Fixed, 95% CI) | 2.87 [0.12, 68.60] |
Differences between protocol and review
We did not conduct a meta‐analysis, therefore were unable to explore the effect of the risk of bias through a sensitivity analysis based on trial quality, as planned. Additional detail on methods of analysis for risk of bias assessment that could be used in future updates of this review, should a meta‐analysis of data be possible, are available in the protocol for this review. Additional detail on methods to address unit of analysis issues, assessment of heterogeneity, assessment of reporting bias, data synthesis, and subgroup and sensitivity analysis that could be used in future updates of this review, should a meta‐analysis of data be possible, are also available in the protocol for this review.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial, randomisation at the level of the individual, conducted over a 3‐year period in 1 acute hospital in New York, US. | |
| Participants | 99 participants with advanced dementia, staged as FAST 6d or greater, hospitalised for an acute illness. 48 in intervention group, 51 in control group | |
| Interventions | Intervention: a palliative care team was established in the hospital, consisting of an experienced clinical nurse specialist and ≥ 1 attending geriatrician(s), who also held academic appointments. The palliative care team conducted a palliative consultation for each participant, visited the participant and discussed participant management with the primary healthcare team in the hospital on a daily basis. The palliative care team also met with family carers or other surrogates when they were available and attempted to arrange meetings after hours or by telephone. The goal of the intervention was to enhance participant comfort. During consultation, options discussed included:
Control: treatment by primary care team without the input of the palliative care team. |
|
| Outcomes | Number of admissions, length of stay and number of deaths in hospital Number of non‐palliative procedures and interventions Decisions to forgo life‐sustaining treatments Decision to adopt a palliative care plan, during hospitalisation and on discharge |
|
| Notes | 1 additional participant was randomised but lost to the study (discharged from the hospital within 24 hours of randomisation) and not included in the analysis. This study was supported by grants from The Greenwall Foundation and The Kornfeld Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After this baseline evaluation, patients were randomly assigned to either the intervention or to the control group." No details given on method of randomisation used. |
| Allocation concealment (selection bias) | Unclear risk | "After this baseline evaluation, patients were randomly assigned to either the intervention or to the control group." No details given on method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given the nature of the intervention, there was no blinding of participants or personnel. We judged the risk of bias due to this lack of blinding to be high for all subjective outcomes, as the primary care team may have made different decisions knowing whether a participant was in the intervention or control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A research assistant blinded to randomization status gathered information from the charts of patients in both arms of the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient was discharged in the first 24 hrs [hours] after randomization and was not readmitted, and was excluded from analysis." |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the Methods section, with the exception of one (do not resuscitate), were reported in the Results. |
| Recruitment bias (cluster trials only) | Low risk | |
| Other bias | High risk | Potential contamination of control participants, who were being treated by the same primary care team that were receiving input from the palliative care team for the intervention participants. |
| Methods | Cluster randomised controlled trial, in 24 nursing homes in the US, randomisation at the nursing home level, with enrolment over a 2‐year period. | |
| Participants | In total, 256 dyads of a resident with advanced dementia and feeding problems and their surrogate were enrolled in the study, 127 in intervention group, 129 in control group. Of these, 90 dyads included a resident with advanced dementia staged as GDS 7 and their surrogate, 46 in intervention group, 44 in control group. | |
| Interventions | Intervention: surrogates received a structured decision aid (printed or audio version) providing information about dementia and feeding options, including feeding for comfort near the end of life, and the outcomes, advantages and disadvantages of feeding tubes or assisted oral feeding. The decision aid also discussed the surrogate's role in decision making. Surrogates reviewed the decision aid during their enrolment interview and received the printed decision aid to take home. Research assistants prompted the surrogates to discuss the decision aid with healthcare providers. Control: surrogates received usual care, including any information typically provided by healthcare providers. |
|
| Outcomes |
For all study participants: Primary outcome: decisional conflict at 3 months, measured by the Decisional Conflict Scale (O'Connor 1995) Secondary outcomes (at 3 months): surrogate knowledge about dementia and feeding options, surrogate‐reported frequency of feeding discussions between surrogate and care provider, and feeding treatment use Secondary outcomes (at 9 months): use of new feeding tubes, number of 'do not tube feed' orders, weight loss and mortality Outcomes included in re‐analysis of subset of participants meeting the inclusion criteria of this review (as requested by review team): Primary outcome: decisional conflict at 3 months, measured by the Decisional Conflict Scale (O'Connor 1995) Secondary outcomes: frequency of feeding discussions between surrogate and care providers and the use of assisted feeding treatments |
|
| Notes | 90/256 (35%) participants had advanced dementia as defined for this systematic review (staged at GDS 7). The study team reran the analysis to produce data for this subset of the study population for this review. Funding source: NIH‐National Institute for Nursing Research RO1 NR009826 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Nursing homes were randomized in pairs matched on variable associated with tube feeding rates… Paired nursing homes were assigned to intervention or control conditions by computerized random number generation conducted by a single investigator (JG)." |
| Allocation concealment (selection bias) | Low risk | "Randomization was completed and allocation concealed prior to enrolment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Information shared with physicians and other health care providers was specific to intervention or control assignment, and direct health care providers were told the general purpose of the study but did not know specifically what the outcome measures were." It was not possible to blind surrogates to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Due to cluster randomization, data collectors were not blinded to group assignment." We judged this lack of blinding to be a high risk of bias for all outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers lost to 3‐month follow‐up in both groups was low (5% and 13%). |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the Methods section were reported and there was no evidence of selective outcome reporting. |
| Recruitment bias (cluster trials only) | High risk | Because of the nature of the intervention, nursing homes were randomised before recruitment of all participants and surrogate dyads. |
| Other bias | Low risk | Baseline imbalance between clusters and cluster effects both accounted for in analysis. |
FAST: Functional Assessment Staging Test; GDS: Global Deterioration Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bergh 2012 | Not a palliative intervention |
| Bonner 2014 | Carers of people with dementia who have not reached the stage of advanced dementia |
| Burns 2009 | Not a palliative intervention |
| De Deyn 2004 | Not a palliative intervention |
| Devanand 2012 | Not a palliative intervention |
| Finkel 1995 | Not a palliative intervention |
| Fleischhacker 1986 | Not a palliative intervention |
| Grossberg 2013 | Not a palliative intervention |
| Hager 2014 | Not a palliative intervention |
| Iwasaki 2007 | Not a palliative intervention |
| Kovach 1996 | Dementia not staged using a validated functional assessment tool |
| Kovach 2006 | > 80% of study participants not advanced dementia; data not available separately for people with advanced dementia |
| Kovach 2012 | > 80% of study participants not advanced dementia; data not available separately for people with advanced dementia |
| Mintzer 2006 | Not a palliative intervention |
| Navratilova 2007 | Not a palliative intervention |
| NCT00921297 | Not a palliative intervention |
| Reinhardt 2004 | > 80% of study participants not advanced dementia; data not available separately for people with advanced dementia |
| Shin 2013 | Not a palliative intervention |
| Street 2000 | Not a palliative intervention |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Improved Dementia End of life care at local Aged care facilities (IDEAL study) |
| Methods | Cluster randomised trial, with residential aged care facility unit of randomisation |
| Participants | Residents with advanced dementia, staged as FAST 6a or greater |
| Interventions | Intervention: case conferencing, with a study‐trained palliative care co‐ordinator at each facility providing ongoing education and mentoring to other staff. Case conferencing for each participant once specific trigger points are reached, to plan further care. Control: usual care |
| Outcomes |
Primary outcomes (family‐rated): Symptom‐related comfort (CAD‐EOLD) last 7 days Symptom management last 90 days (SM‐EOLD) Family satisfaction with care during the last 90 days of life (SWC‐EOLD) Secondary outcomes (nurse‐rated): Symptom‐related comfort 7 days (CAD‐EOLD) Symptom manage 90 days (SM‐EOLD) QoL (QUALID) EQ‐5D‐5L Process outcomes: Person‐centred approach to care (PCECAT) Staff attitudes to, knowledge and confidence in providing palliative/EOL care (PCADQ) Quality of EOL care: acute care episodes, aggressive medical intervention |
| Starting date | 2012 |
| Contact information | Dr Tim Luckett, University of Technology Sydney (UTS) (t.luckett@unsw.edu.au) |
| Notes | Recruitment closed, follow‐up completed |
| Trial name or title | Improve Quality of Care and Quality of Dying in Advanced Dementia |
| Methods | Controlled before‐and‐after study |
| Participants | Nursing home residents with advanced dementia, staged as GDS 7 |
| Interventions | Intervention: training nurses, physicians and families on symptomatic care approach for end‐stage pneumonia and feeding difficulties. Main focus is on early detection of pain, early mouth care and family support for decision‐making. There is a nurse champion in each home. Control: Usual care |
| Outcomes | Quality of EOL care (FPCS), quality of dying (CAD‐EOL) |
| Starting date | 2011 |
| Contact information | Marcel Arcand, Universite de Sherbrooke, Quebec, Canada (marcel.arcand@usherbrooke.ca) |
| Notes | Recruitment closed, follow‐up complete |
| Trial name or title | Feedback on End‐of‐Life Care in Dementia: the FOLlow‐Up Study |
| Methods | Randomised controlled trial, nursing home the unit of randomisation |
| Participants | Country: Netherlands Setting: 18 nursing homes Participants: family carers of nursing home residents with dementia who died on a psychogeriatric ward, approximately 30% of whom had advanced dementia at time of death. |
| Interventions | Intervention: after death of a resident with dementia, nursing homes invited the family to provide feedback on care using EOLD instruments. 2 different audit and feedback strategies were used to communicate the family feedback to staff. Control: administration of the EOLD instruments without any feedback to staff, in addition to usual care. |
| Outcomes | Primary outcomes: satisfaction with care (EOLD‐SWC); comfort in dying (EOLD‐CAD) Secondary outcome: process evaluation |
| Starting date | May 2012 |
| Contact information | Jenny van der Steen |
| Notes | Data collected over 20 months. |
| Trial name or title | Goals of Care: a Nursing Home Trial of Decision Support for Advanced Dementia (GOC) |
| Methods | Cluster randomised controlled trial |
| Participants | Nursing home residents with dementia staged as GDS 5 to 7 Nursing homes Estimated enrolment 300 |
| Interventions | Intervention: aid video viewed by surrogate of resident with advanced dementia in nursing home, following by a care plan meeting between surrogate and interdisciplinary nursing home team Control: attention control information on dementia care |
| Outcomes | Primary outcome: composite score of:
Secondary outcomes: number (out of 10) of palliative care domains in care plan; hospice referral; hospitalisations; family‐rated: satisfaction with care (SWC‐EOLD), comfort in dying (CAD‐EOLD), QoL (QUALID), quality of dying (QOD‐LTC), frequency of communication with providers |
| Starting date | May 2012 |
| Contact information | Laura C Hanson, MD, MPH, University of North Carolina, US (laura_hanson@med.unc.edu) |
| Notes | Analysis by severity of dementia planned |
| Trial name or title | Educational Video to Improve Nursing Home Care in End‐Stage Dementia (EVINCE) |
| Methods | Cluster randomised controlled trial, nursing home the unit of randomisation |
| Participants | People with advanced dementia, staged as GDS 7 |
| Interventions | Intervention: advance care planning video for proxy, identifying 3 possible levels of care: intensive, basic and comfort Control: usual advanced care planning process as per nursing home |
| Outcomes | Primary outcome: documented decision to forgo hospitalisation at 6 months Secondary outcomes: preference for level of care (comfort vs other), decisions to forgo burdensome treatments, receipt of burdensome treatments, subgroup analysis: decisions since start of study to forgo hospitalisations |
| Starting date | March 2013 |
| Contact information | Susan Mitchell, Boston, USA (smitchell@hsl.harvard.edu) |
| Notes | Target study completion February 2018 |
| Trial name or title | Advanced Care Planning |
| Methods | Cluster randomised trial, nursing home the unit of randomisation |
| Participants | Nursing home residents with dementia, staged as FAST 6 or 7 |
| Interventions | Intervention: family education on comfort care at EOL for people with dementia, an advanced care planning nurse facilitator, meetings with family Control: usual care |
| Outcomes | Primary outcome: decisional conflict at 6 weeks (Decisional Conflict Scale) Secondary outcomes: proxy satisfaction with care (FPCS), proxy anxiety and depression (GHQ‐12), comfort of resident at EOL (QODLTC), number of unnecessary hospitalisations |
| Starting date | 2015 |
| Contact information | Kevin Brazil (k.brazil@qub.ac.uk) |
| Notes |
CAD‐EOL: Comfort Assessment in Dying at the End of Life; CAD‐EOLD: Comfort Assessment in Dying at the End of Life with Dementia; SM‐EOLD: Symptom Management at the End Of Life in Dementia; EOL: end of life; EOLD: End‐of‐Life in Dementia; FAST: Functional Assessment Staging Test; FPCS: Family Perception of Care Scale; GHQ‐12: 12‐item General Health Questionnaire; QOC: quality of care; QOD‐LTC: Quality of Dying in Long‐Term Care; QoL: quality of life; QUALID: Quality of Life in Late‐Stage Dementia; PCADQ: Palliative Care for Advanced Dementia Questionnaire; PCECAT: Person‐centred Environment and Care Assessment Tool; SWC‐EOLD: End‐of‐Life in Dementia scales ‐ Satisfaction With Care.
Contributions of authors
EM assessed studies for inclusion, completed data extraction and drafted the protocol and the review.
SC assessed studies for inclusion, completed data extraction and commented on all sections through to the final review.
KF, EOS and EL contributed to decisions on inclusion where consultation was required and commented on all drafts through to the final review.
DC commented on drafts of the review.
DD provided a methodological perspective, advice on writing the protocol and review, support with the 'Summary of findings' table and commented on all drafts through to the final review.
All authors contributed to the outcome prioritisation process.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The Health Research Board, Ireland.
Cochrane Review Training Fellowship (recipient: Edel Murphy)
-
NIHR, UK.
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
We have no known conflicts of interest to declare.
New
References
References to studies included in this review
- Ahronheim JC, Morrison RS, Morris J, Baskin S, Meier DE. Palliative care in advanced dementia: a randomized controlled trial and descriptive analysis. Journal of Palliative Medicine 2000;3(3):265‐73. [] [DOI] [PubMed] [Google Scholar]; Baskin SA, Morris J, Ahronheim JC, Meier DE, Morrison RS. Barriers to obtaining consent in dementia research: implications for surrogate decision‐making. Journal of American Geriatric Society 1998;46(3):287‐90. [] [DOI] [PubMed] [Google Scholar]; 4479310
- Hanson LC, Carey TS, Caprio AJ, Lee TJ, Ersek M, Garrett J, et al. Improving decision making for feeding options in advanced dementia: a randomized, controlled trial. Journal of the American Geriatric Society 2011;59(11):2009‐16. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; Hanson LC, Gilliam R, Lee TJ. Successful clinical trial research in nursing homes: the improving decision‐making study. Clinical Trials 2010;7(6):735‐43. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479313
References to studies excluded from this review
- Bergh S, Selbaek G, Engedal K. Discontinuation of antidepressants in people with dementia and neuropsychiatric symptoms (DESEP study): double blind, randomised, parallel group, placebo controlled trial. BMJ 2012;344:e1566. [] [DOI] [PubMed] [Google Scholar]; 4479316
- Bonner G, Wang E, Wilkie D, Ferrans C, Dancy B, Watkins Y. Advance care treatment plan (ACT‐Plan) for African American family caregivers: a pilot study. Dementia 2014;13(1):79‐95. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479318
- Burns A, Bernabei R, Bullock R, Cruz Jentoft AJ, Frölich L, Hock C, et al. Safety and efficacy of galantamine (Reminyl) in severe Alzheimer's disease (the SERAD study): a randomised, placebo‐controlled, double‐blind trial. Lancet Neurology 2009;8(1):39‐47. [] [DOI] [PubMed] [Google Scholar]; 4479320
- Deyn PP, Martın‐Carrasco M, Deberdt W, Jeandel C, Hay DP, Feldman PD. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19:115‐26. [] [DOI] [PubMed] [Google Scholar]; 4479322
- Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, Pena D. Relapse risk after discontinuation of risperidone in Alzheimer's disease. New England Journal of Medicine 2012;367(16):1497‐507. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479324
- Finkel SI, Lyons JS, Anderson RL, Sherrell K. A randomized, placebo‐controlled trial of thiothixene in agitated, demented nursing home patients. International Journal of Geriatric Psychiatry 1995;10:129‐36. [] [Google Scholar]; 4479326
- Fleischhacker WW, Buchgeher A, Schubert H. Memantine in the treatment of senile dementia of the Alzheimer type. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 1986, (1):87‐93. [] [DOI] [PubMed] [Google Scholar]; 4479328
- Grossberg GT, Manes F, Allegri RF, Gutierrez‐Robledo LM, Gloger S, Xie L, et al. The safety, tolerability, and efficacy of once‐daily memantine (28 mg): a multinational, randomized, double‐blind, placebo‐controlled trial in patients with moderate‐to‐severe Alzheimer's disease taking cholinesterase inhibitors. CNS Drugs 2013;27:469‐78. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479330
- Hager K, Baseman AS. Nye JS, Brashear HR, Han J, Sano M, et al. Effects of galantamine in a 2‐year, randomized, placebo‐controlled study in Alzheimer's disease. Neuropsychiatric Disease and Treatment 2014;10:391‐401. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479332
- Iwasaki K, Kato S, Monma Y, Niu K, Ohrui T, Okitsu R, et al. A pilot study of Banxia Houpu Tang, a traditional Chinese medicine, for reducing pneumonia risk in older adults with dementia. Journal of the American Geriatric Society 2007;55:2035‐40. [] [DOI] [PubMed] [Google Scholar]; 4479334
- Kovach CR, Wilson SA, Noonan PE. The effects of hospice interventions on behaviors, discomfort, and physical complications of end stage dementia nursing home residents. American Journal of Alzheimer's Disease 1996;11(4):7‐15. [] [Google Scholar]; 4479336
- Kovach CR, Logan BR, Noonan PE, Matovina Schlidt A, Smerz J, Simpson M, et al. Effects of the Serial Trial intervention on discomfort and behavior of nursing home residents with dementia. American Journal of Alzheimer's Disease & Other Dementias 2006;21(3):247‐55. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479338
- Kovach CR, Simpson MR, Joosse L, Logan BR, Noonan PE, Reynolds SA, et al. Comparison of the effectiveness of two protocols for treating nursing home residents with advanced dementia. Research in Gerontological Nursing 2012;5(4):251‐63. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479340
- Mintzer J, Greenspan A, Caers I, Hove I, Kushner S, Weiner M, et al. Risperidone in the treatment of psychosis of Alzheimer disease: results from a prospective clinical trial. American Journal of Geriatric Psychiatry 2006;14(3):280‐91. [] [DOI] [PubMed] [Google Scholar]; 4479342
- Navratilova M, Jarkovsky J, Ceskova E, Leonard B, Sobotka L. Alzheimer disease: malnutrition and nutritional support. Clinical and Experimental Pharmacology and Physiology 2007;34:S11‐3. [] [Google Scholar]; 4479344
- NCT00921297. Cataract removal and Alzheimer's disease. clinicaltrials.gov/show/NCT00921297 Date first received: 15 June 2009. [] ; 4479346
- Reinhardt JP, Chichin D, Posner L, Kassabian S. Vital conversations with family in the nursing home: preparation for end‐stage dementia care. Journal of Social Work in End‐of‐Life & Palliative Care 2014;10(2):112‐26. [] [DOI] [PubMed] [Google Scholar]; 4479348
- Shin CS. A randomized placebo‐controlled trial of quetiapine for the treatment of behavioral and psychological symptom of dementia in Alzheimer's disease patients. Journal of the Neurological Sciences 2013:e294‐5. [] [Google Scholar]; 4479350
- Street JS, Clark WS, Gannon KS, Cummngs JL, Bymaster FP, Tamura RN, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer's disease in nursing care facilities. Archives of General Psychiatry 2000;57(10):968‐76. [] [DOI] [PubMed] [Google Scholar]; 4479352
References to ongoing studies
- Agar M, Beattie E, Luckett T, Phillips J, Luscombe G, Goodall S, et al. Pragmatic cluster randomised controlled trial of facilitated family case conferencing compared with usual care for improving end of life care and outcomes in nursing home residents with advanced dementia and their families: the IDEAL study protocol. BioMed Central Palliative Care 2015;14:63. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479354
- Arcand M, Verreault R, Mission L. A successful intervention to improve quality of end‐of‐life care (QOC) and quality of dying (QOD) for patients with advanced dementia. European Association for Palliative Care World Conference Abstracts; 2015 May 8‐10; Copenhagen, Denmark. 2015. [] [Google Scholar]; 4479356
- Boogaard JA, Soest‐Poortvliet MA, Anema JR, Achterberg WP, Hertogh CMPM, Vet HCW, et al. Feedback on end‐of‐life care in dementia: the study protocol of the FOLlow‐up project. BioMed Central Palliative Care 2013;12:29. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479358
- Einterz SF, Gilliam R, Lin F‐C, McBride JM, Hanson LC. Development and testing of a decision aid on goals of care for advanced dementia. Journal of American Medical Directors Association 2014;15(4):251‐55. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 4479360
- NCT01774799. Educational video to improve nursing home care in end‐stage dementia (EVINCE). www.clinicaltrials.gov/ct/show/NCT01774799 Date first received: 21 January 2013. [] ; 4479362
- NCT02211287. Promoting informed decision making through advance care planning. www.clinicaltrials.gov/ct2/show/NCT02211287 Date first received: 6 August 2014. [] ; 4479364
Additional references
- Allan K, Killick J. Communication and relationships: an inclusive social world. In: Downs M, Bowers B editor(s). Excellence in Dementia Care: Research into Practice. 2nd Edition. McGraw Hill Education, Open University Press, 2014:240‐56. [Google Scholar]
- Allen RS, Kwak J, Lokken KL, Haley WE. End‐of life issues in the context of Alzheimer's disease. Alzheimers Care Quarterly 2003;4(4):312‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association. 2014 Alzheimer's disease facts and figures. Alzheimer's & Dementia 2014;10(2):e47‐92. [DOI] [PubMed] [Google Scholar]
- Australian Government Department of Health and Ageing. Guidelines for a palliative approach in residential aged care. Enhanced version ‐ May 2006. www.nhmrc.gov.au/_files_nhmrc/publications/attachments/pc29.pdf (accessed 14 March 2014).
- Bausewein C, Daveson B, Benalia H, Simon ST, Higginson IJ. Outcome measurement in palliative care: the essentials. 2011. www.eapcnet.eu/LinkClick.aspx?fileticket=‐T62WTgTHtU%3d&tabid=1577 (accessed 13 August 2014).
- Brayne C, Gao L, Dewey M, Matthews FE, Medical Research Council Cognitive Function and Ageing Study Investigators. Dementia before death in ageing societies ‐ the promise of prevention and the reality. PLoS Medicine 2006;3(10):e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Archives of Neurology 2002;59(11):1764‐7. [DOI] [PubMed] [Google Scholar]
- Brown MA, Sampson EL, Jones L, Barron AM. Prognostic indicators of 6‐month mortality in elderly people with advanced dementia: a systematic review. Palliative Medicine 2012;27(5):389‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill S, O'Shea E, Pierce M. Creating excellence in dementia care: a research review for Ireland's National Dementia Strategy. 2012. www.icsg.ie/sites/www.icsg.ie/files/personfiles/creating_excellence_in_dementia_care2012_0.pdf. Dublin, (accessed 1 Dec 2016).
- Candy B, Jones L, Varagunam M, Speck P, Tookman A, King M. Spiritual and religious interventions for well‐being of adults in the terminal phase of disease. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD007544.pub2] [DOI] [PubMed] [Google Scholar]
- Capon A, Pavoni N, Mastromattei A, Lallo D. Pressure ulcer risk in long‐term units: prevalence and associated factors. Journal of Advanced Nursing 2007;58(3):263‐72. [DOI] [PubMed] [Google Scholar]
- Chan RJ, Webster J. End‐of‐life care pathways for improving outcomes in caring for the dying. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD008006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agata E, Mitchell SL. Patterns of antimicrobial use among nursing home residents with advanced dementia. Archives of Internal Medicine 2008;168(4):357‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E, Higginson IJ (editors). Better Palliative Care for Older People. Copenhagen: World Health Organization, Regional Office for Europe, 2004. [Google Scholar]
- Giulio P, Toscani F, Villani D, Brunelli C, Gentile S, Spadin P. Dying with advanced dementia In long‐term care geriatric institutions: a retrospective study. Journal of Palliative Medicine 2008;11(7):1023‐8. [DOI] [PubMed] [Google Scholar]
- European Association for Palliative Care. Definition of palliative care. www.eapcnet.eu/Corporate/AbouttheEAPC/Definitionandaims.aspx. Europe, 1998 (last accessed 5 January 2016).
- Engelberg R, Downey L Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end‐of‐life care. Journal of Palliative Medicine 2006;9(5):1086‐1098. [DOI] [PubMed] [Google Scholar]
- Cochrane Effective Practice and Organisation of Care (EPOC) Group. What study designs should be included in an EPOC review?. epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/EPOC%20Study%20Designs%20About.pdf (accessed 8 April 2014).
- Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. New England Journal of Medicine 2010;362(13):1173‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens JL, Jones RN, Shaffer ML, Kiely DK, Mitchell SL. Survival and comfort after treatment of pneumonia in advanced dementia. Archives of Internal Medicine 2010;170(13):1102‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost‐effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD007760.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster University. GRADEpro. Hamilton (ON): McMaster University, 2014.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Kolliakou A, Davies EA, Froggatt K, Higginson IJ. Interventions for improving palliative care for older people living in nursing care homes. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007132.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Higginson IJ, Finlay IG, Goodwin DM, Hood K, Edwards AG, Cook A, et al. Is there evidence that palliative care teams alter end‐of‐life experiences of patients and their caregivers?. Journal of Pain and Symptom Management 2003;25(2):150‐68. [DOI] [PubMed] [Google Scholar]
- Houttekier D, Cohen J, Bilsen J, Addington‐Hall J, Onwuteaka‐Philipsen BD, Deliens L. Place of death of older persons with dementia. A study in five European countries. Journal of the American Geriatric Society 2010;58(4):751‐6. [DOI] [PubMed] [Google Scholar]
- Hubbard G, Cook A, Tester S, Downs M. Beyond words: older people with dementia using and interpreting non‐verbal behaviour. Journal of Aging Studies 2002;16:155‐67. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry 1982;140:566‐72. [DOI] [PubMed] [Google Scholar]
- Kim KY, Yeaman PA, Keene RL. End‐of‐life care for persons with Alzheimer's disease. Psychiatric Services 2005;56(2):139‐41. [DOI] [PubMed] [Google Scholar]
- Krouse RS, Rosenfeld KE, Grant M, Aziz N, Byock I, Sloan J, et al. Palliative care research: issues and opportunities. Cancer Epidemiology Biomarkers and Prevention 2004;13:337‐9. [PubMed] [Google Scholar]
- Lamas D, Rosenbaum L. Painful inequities ‐ palliative care in developing countries. New England Journal of Medicine 2012;366(3):199‐201. [DOI] [PubMed] [Google Scholar]
- Mitchell SL, Morris JN, Park PS, Fries BE. Terminal care for persons with advanced dementia in the nursing home and home care settings. Journal of Palliative Medicine 2004;7(6):808‐16. [DOI] [PubMed] [Google Scholar]
- Mitchell SL, Kiely DK, Miller SC, Conor SR, Spence C, Teno JM. Hospice care for patients with dementia. Journal of Pain and Symptom Management 2007;34(1):7‐16. [DOI] [PubMed] [Google Scholar]
- Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. New England Journal of Medicine 2009;361(16):1529‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SL, Black BS, Ersek M, Hanson LC, Miller SC, Sachs GA, et al. Advanced dementia: state of the art and priorities for the next decade. Annals Internal Medicine 2012;156(1 Pt 1):45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RS, Siu AL. Survival in end‐stage dementia following acute illness. JAMA 2000;284(1):47‐52. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Siu AL. A comparison of pain and its treatment in advanced dementia and cognitively intact patients with hip fracture. Journal in Pain and Symptom Management 2000;19(4):240‐8. [DOI] [PubMed] [Google Scholar]
- National Council for Palliative Care. Exploring Palliative Care for People with Dementia: a Discussion Document. London: National Council for Palliative Care, 2006:1‐23. [Google Scholar]
- O'Connor AM. User manual ‐ Decisional Conflict Scale, 1993 [updated 2010]. decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf (accessed 26 November 2016).
- O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making 1995;15(1):25‐30. [DOI] [PubMed] [Google Scholar]
- Parker D, Hodgkinson B. A comparison of palliative care outcome measures used to assess the quality of palliative care provided in long‐term care facilities: a systematic review. Palliative Medicine 2011;25(1):5‐20. [DOI] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and meta‐analysis. Alzheimers & Dementia 2013;9(1):63‐75 e2. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. American Journal of Psychiatry 1982;139(9):1136‐9. [DOI] [PubMed] [Google Scholar]
- Reisberg B. Functional assessment staging (FAST). Psychopharmacology Bulletin 1988;24(4):653‐9. [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Ryan T, Gardiner C, Bellamy G, Gott M, Ingleton C. Barriers and facilitators to the receipt of palliative care for people with dementia: the views of medical and nursing staff. Palliative Medicine 2012;26(7):879‐86. [DOI] [PubMed] [Google Scholar]
- Scherder E, Oosterman J, Swaab D, Herr K, Ooms M, Ribbe M, et al. Recent developments in pain in dementia. BMJ 2005;330(7489):461‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaji KS. Dementia care in developing countries: the road ahead. Indian Journal of Psychiatry 2009;51(Suppl 1):S5‐7. [PMC free article] [PubMed] [Google Scholar]
- Shuster JL Jr. Palliative care for advanced dementia. Clinics in Geriatric Medicine 2000;16(2):373‐86. [DOI] [PubMed] [Google Scholar]
- Torke AM, Holtz LR, Hui S, Castelluccio P, Connor S, Eaton MA, et al. Palliative care for patients with dementia: a national survey. Journal of the American Geriatric Society 2010;58(11):2114‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health, London. UK End of Life Care Strategy, 2008. www.gov.uk/government/uploads/system/uploads/attachment_data/file/136431/End_of_life_strategy.pdf (accessed 28 November 2016).
- Steen JT, Radbruch L, Hertogh CM, Boer ME, Hughes JC, Larkin P, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliative Medicine 2014;28(3):197‐209. [DOI] [PubMed] [Google Scholar]
- Volicer L, Collard A, Hurley A, Bishop C, Kern D, Karon S. Impact of special care unit for patients with advanced Alzheimer's disease on patients' discomfort and costs. Journal of the American Geriatric Society 1994;42(6):597‐603. [DOI] [PubMed] [Google Scholar]
- Volicer L, Hurley AC, Blasi ZV. Scales for the evaluation of end‐of‐life care in dementia. Alzheimer Disease and Associated Disorders 2001;15(4):194‐200. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Dementia: a public health priority. apps.who.int/iris/bitstream/10665/75263/1/9789241564458_eng.pdf?ua=1 (accessed 8 April 2014).
References to other published versions of this review
- Murphy E, Froggatt K, Connolly S, O'Shea E, Sampson EL, Casey D, Devane D. Palliative care interventions in advanced dementia. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011513] [DOI] [PMC free article] [PubMed] [Google Scholar]


