Abstract
The fetus is dependent on delivery of fatty acids (FAs) by the syncytiotrophoblast, the transporting epithelium of the human placenta. Obese pregnant women have dyslipidemia; however, whether obesity impacts placental lipid transport and metabolism remains to be fully established. Palmitoleic acid (POA), an FA with anti-inflammatory and insulin-sensitizing properties, is synthesized from palmitic acid (PA) catalyzed by stearoyl–coenzyme A desaturase (SCD) activity. We hypothesized that the uptake and incorporation of FAs and POA synthesis are reduced in primary human trophoblasts (PHTs) isolated from pregnancies complicated by maternal obesity. Villous cytotrophoblasts were isolated from 7 placentas of obese [body mass index (BMI) = 37.5 ± 1.9] and 12 normal (BMI = 23.6 ± 0.6) mothers. FA uptake and incorporation were assessed using uniformly labeled (U[13C])–FA mixtures of PA, oleic acid (OA), linoleic acid, and docosahexaenoic acid. Cellular [13C] FAs were quantified both in total cellular lipids and in lipid classes by GC-MS. Uptake and incorporation of [13C] FAs in total cellular lipids were not different in PHTs isolated from obese mothers compared with normal mothers. Only the concentration of OA was increased in the triglyceride fraction (P < 0.05) if the mother was obese. We found an isotopic enrichment of POA after U[13C]-PA treatment, demonstrating SCD activity in PHT cells. Labeled POA content and the POA:PA ratio were significantly lower in PHTs isolated from placentas of obese mothers compared with normal, healthy controls. Decreased syncytiotrophoblast POA synthesis may contribute to insulin resistance and low-grade inflammation in the mother, placenta, or fetus (or a combination of the 3) in pregnancies complicated by obesity.—Ferchaud-Roucher, V., Barner, K., Jansson, T., Powell, T. L. Maternal obesity results in decreased syncytiotrophoblast synthesis of palmitoleic acid, a fatty acid with anti-inflammatory and insulin-sensitizing properties.
Keywords: POA synthesis, pregnancy, placenta, lipids
Almost two-thirds of American women now enter pregnancy either overweight or obese (1, 2), conditions which are associated with a range of short- and long-term adverse maternal and fetal outcomes (3). For example, obese women are more likely to deliver an infant who is large for gestational age (4, 5), has increased adiposity (6), or is insulin resistant at birth (or a combination of the 3) (7). Babies born to obese mothers, in particular if they were large at birth, have an increased risk to develop obesity, diabetes, and cardiovascular disease in childhood and later in life (8–10).
Lipolytic activity in maternal adipose tissue is increased in the third trimester, promoting the release of fatty acids (FAs), which are primarily incorporated into triglycerides (TGs) by the maternal liver, leading to the markedly higher circulating TG levels toward the end of pregnancy (11). This physiologic hyperlipidemia is exacerbated in obese pregnant women and may be associated with lipotoxicity, leading to maternal endothelial dysfunction (12).
The composition of FAs delivered to the fetus is determined by both the maternal circulating lipid levels and FA uptake and metabolism by the syncytiotrophoblast, the transporting epithelium of the human placenta. Trophoblasts are believed to take up nonesterified FAs (NEFAs) released from circulating maternal TGs and phospholipids (PLs) through hydrolysis by maternal lipoprotein lipase, endothelial lipase (13), and phospholipase A2. Preferential transfer of some long-chain polyunsaturated FAs (LCPUFAs) across the syncytiotrophoblast has been described and can be attributable to differences in the affinity of LCPUFAs for specific FA transport proteins and FA binding proteins that are responsible for their uptake. Once taken up, FAs have 3 potential cellular fates: β-oxidation by the mitochondria, re-esterification (which occurs in the endoplasmic reticulum), or transfer to the fetus across the basal plasma membrane of the syncytiotrophoblast. The mechanisms underlying uptake (the first step of transplacental delivery of lipids) and cellular esterification of FAs by the syncytiotrophoblast remain poorly understood. Moreover, the impact of maternal obesity on placental lipid transfer to the fetus remains to be fully established.
Maternal obesity has been reported to be associated with an increased placental lipid accumulation in the cellular TG fraction (14). This high placental lipid content was not explained by an increased FA uptake (14, 15) but rather by an alteration in lipid esterification and down-regulation in FA oxidation (16), suggesting that the placenta of obese mothers may be modulating delivery of lipids to the fetus. In most studies, only the total placental lipid content has been analyzed. Because the concentration and composition of FAs in distinct cellular lipid fractions varies significantly under changing physiologic conditions and in disease states, it is important to understand how maternal obesity influences placental incorporation of FAs in different lipid classes.
Palmitoleic acid (C16:1n-7, POA) and oleic acid (C18:1n-9, OA), 2 monounsaturated FAs (MUFAs), are synthesized from palmitic acid (PA) and stearic acid (SA), respectively. The reaction is catalyzed by the rate-limiting enzyme δ-9 desaturase, or stearoyl–coenzyme A desaturase (SCD). Studies by Ntambi et al. (17) employing global SCD1 knockout mice and, more recently, by Vázquez-Vela et al. (18) using mice lacking SCD1 specifically in the liver have indicated that endogenous MUFA synthesis contributes to whole-body energy homeostasis. Mice lacking SCD1 are lean and protected against induction of obesity by diet (17) or leptin deficiency (19). OA and POA are both products of SCD1 and have distinct metabolic properties. POA is considered to be a strong indicator for de novo lipogenesis and exerts lipokine effects by strongly stimulating insulin sensitivity in muscles and suppressing hepatosteatosis in mice (20, 21). POA also reverses proinflammatory gene expression in bone marrow cells and macrophage polarization through the activation of AMPK (22, 23). Finally, POA has been associated with increased insulin sensitivity and with a lower incidence of diabetes in humans (24). Currently, there are no reports about a potential role of POA in lipid metabolism in the human placenta.
We hypothesized that the uptake and incorporation of FAs and POA synthesis are reduced in primary human trophoblast (PHT) cells isolated from pregnancies complicated by maternal obesity. We developed a novel methodology to study the uptake and metabolism of 4 representative NEFA species in PHT cells using a physiologic mixture of 4 [13C] stable isotope–labeled FAs [PA, OA, linoleic acid (LA), and docosahexaenoic acid (DHA)]. We determined the incorporation of these 4 representative [13C]-FAs into 3 complex lipid forms: cholesterol esters (CEs), TGs, and PLs in PHT cells isolated from placentas of normal, healthy pregnancies and those complicated by obesity. We also studied the synthesis of POA from [13C] PA. This study advances our understanding of the mechanisms involved in placental FA uptake and metabolism and how these are altered by maternal obesity and identifies novel factors in the delivery of FAs to the fetus.
MATERIALS AND METHODS
Study subjects for placenta collection
Women were recruited to donate their placenta to a data/biorepository for use in multiple research studies. Informed written consent was obtained from all participants for collection of placental tissue and for the use of their protected health information under a protocol approved by the Institutional Review Board at the University of Colorado–Denver (Colorado Multiple Institutional Review Board, 14-1073). Inclusion criteria for the repository included a range of prepregnancy or early pregnancy body mass index (BMI), ultrasound confirmation of gestational age at 14–18 wk, singleton pregnancy, and maternal age of 18–45 yr. Exclusion criteria included concurrent inflammatory, vascular, or metabolic disease; current use of tobacco, street drugs, or medications; fetal malformations; history of pregnancy loss; or preterm delivery. Maternal and infant characteristics of the subjects selected for the current study are summarized in Table 1 and demonstrate that maternal BMI was the key factor that distinguished the 2 groups. Anonymized tissue was transferred to the laboratory for isolation of cytotrophoblast cells immediately after delivery.
TABLE 1.
Clinical characteristics of subjects
| Variable | Time course | Control | Obese |
|---|---|---|---|
| Subjects (n) | 5 | 7 | 7 |
| Maternal BMI (kg/m2) | 23.4 ± 1.0 | 23.7 ± 0.8 | 37.5 ± 1.9* |
| Age at delivery (yr) | 33.6 ± 1.4 | 32.3 ± 1.5 | 32.3 ± 1.7 |
| Gestational age (wk) | 39.1 ± 0.1 | 39.2 ± 0.1 | 39.3 ± 0.1 |
| Placental weight (g) | 727.2 ± 70.2 | 738.7 ± 65.5 | 742.6 ± 70.2 |
| Birth weight (g) | 3502.8 ± 92.4 | 3483.7 ± 108.8 | 3517.0 ± 180.8 |
| Infant sex | 5 males | 6 males/1 female | 2 males/5 females |
Data are presented as means ± sem. Time course (normal BMI) = PHT cells used for U[13C]-FA model. Obese vs. control = PHT cells used for studying the placental lipid changes in pregnancy complicated by obesity. *P < 0.05 (Mann-Whitney U test).
Culture of PHT cells and uniformly labeled[13C]-FA uptake and metabolism
Villous cytotrophoblast cells were isolated and purified as previously described in refs. 25 and 26. Briefly, cells were isolated using DNase and trypsin digestion and purification on a Percoll gradient. PHTs were plated at a density of 4 × 106 per 60-mm dish and cultured for 90 h in medium containing Ham’s F-12 and high-glucose DMEM (1:1, v/v), 10% fetal bovine serum (S11550; Atlanta Biologicals, Flowery Branch, GA, USA), and antibiotics (penicillin, gentamicin, and streptomycin) according to a well-established protocol (27–29).
At 66 h of culture, trophoblast cells were treated with a combination of uniformlylabeled [13C] containing (U[13C])–PA (95 µM), U[13C]-OA (95 µM), U[13C]-LA (95 µM), and U[13C]-DHA (15 µM) (Cambridge Isotope Laboratories, Tewksbury, MA, USA). Each labeled FA was solubilized in 10% FA-free bovine serum albumin (BSA) and added to culture medium containing 10% lipoprotein-depleted fetal bovine serum (Kalen Biomedical, Germantown, MD, USA). These FAs were selected to represent the 4 main types of FAs: PA, a saturated FA (SFA); OA, a MUFA; LA, an n-6 essential polyunsaturated FA; and DHA, an n-3 LCPUFA in physiologic concentrations found in maternal circulation at the end of normal pregnancy. Control cells were treated in the same conditions by adding an equal volume of FA-free BSA to the culture medium. Cells were incubated at 37°C with 5% CO2/atmosphere air and studied over a time course from 30 min to 36 h. Cells were harvested as previously described (30), and at each time point, cells were rapidly and carefully washed 4 times in ice-cold PBS, scraped using a rubber-tipped spatula in 0.9% NaCl, and stored at −80°C until analysis.
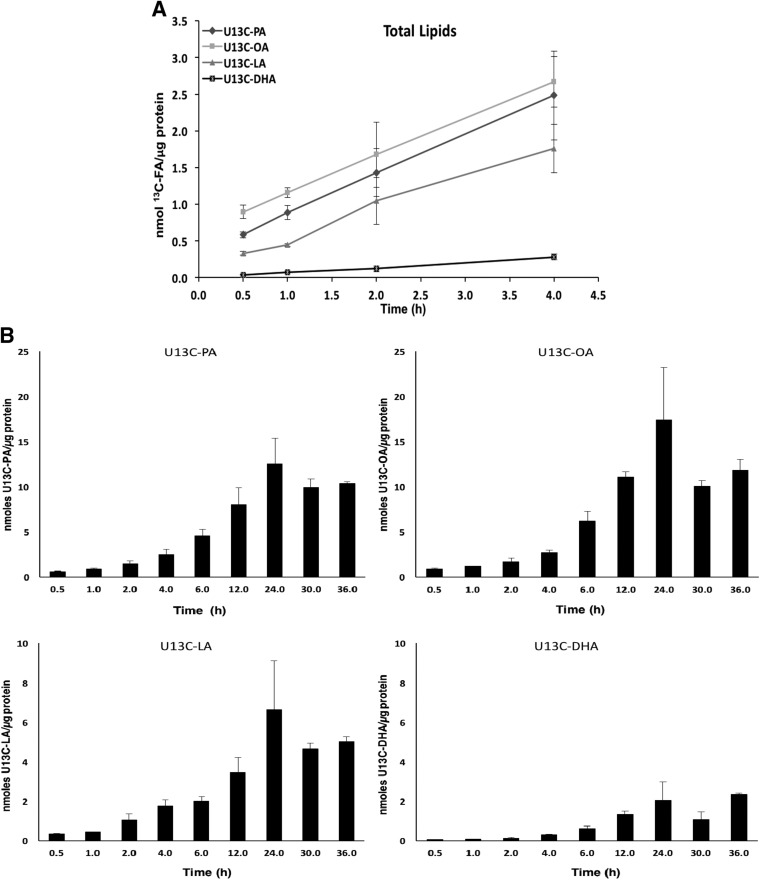
After validation of the experimental model using a U[13C]-FA mixture incubated with PHT cells isolated from placentas of 5 normal, healthy pregnant women (Fig. 1), this method was applied in PHT cells isolated from placentas from pregnant women who were obese or who had a normal BMI (n = 7 in each group). Examination of the uptake and incorporation of individual FAs over time indicated that 2 h represented the initial uptake phase and 30 h represented steady-state incorporation of the 4 FAs studied.
Figure 1.
U[13C]-FA concentration in total lipid pool in cultured PHT cells. Change over time in absolute concentrations of labeled PA, OA, LA and DHA expressed as nanomoles of FA per microgram of protein in total lipid pool extracted from PHT cells (n = 3 to 5 for each time point, means + sem). A) Uptake of U[13C]-FAs over the first 4 h of incubation. B) Total U[13C]-FA content over 36 h of incubation.
Characterization of cultured PHT cells
Release of human chorionic gonadotropin (hCG) from trophoblast cells was used as a biochemical marker of differentiation. Concentrations of hCG in the cell culture medium were measured (β-hCG ELISA; DRG Instruments, Marburg, Germany) at 18, 42, 66, and 90 h after plating the cells (26). hCG secretion into the culture medium increased more than 4-fold between 18 and 66 h (unpublished results), confirming differentiation, syncytialization, and cell viability (27), and remained stable for the duration of the incubation with [13C] FAs.
Western blotting
Western blot was performed as previously described (31, 32) using a precast gel system (Bio-Rad, Hercules, CA, USA). Total protein content of trophoblast cells collected after 90 h of culture was determined using a bicinchoninic acid (BCA) assay (Pierce BCA Protein Assay Kit; Thermo Fisher Scientific, Waltham, MA, USA). Ten micrograms of total protein was loaded and separated on Bis-Tris Gels (4–20%; Bio-Rad). Lysate of human embryonic kidney 293 cells (HEK293, ab7902; Abcam, Cambridge, MA, USA) served as a positive control. Electrophoresis was performed at 120 V for 20 min and then at a constant 150 V for 1 h. Proteins were transferred onto a PVDF membrane (Bio-Rad) overnight at 4°C and a constant 35 V. The membrane was first stained for total protein with Amido Black stain (MilliporeSigma, Burlington, MA, USA) and then cleared and blocked for 1 h in 5% BSA in Tris-buffered saline (TBS)–Tween. Incubation with primary antibodies [anti-SCD1, diluted 1:500, ab39969; anti–carnitine palmitoyltransferase (CPT) 1b, diluted 1:1000, ab134988; anti-CPT2, diluted 1:1000, ab181114; all antibodies purchased from Abcam] was carried out overnight at 4°C in 5% BSA in TBS-Tween except for CPT2, for which 5% milk in TBS-Tween was used. The washed membranes were incubated for 1 h at room temperature with secondary antibody (peroxidase-labeled anti-rabbit IgG; Cell Signaling Technology, Danvers, MA, USA) diluted at 1:10000 for SCD1 and CPT1b and 1:2000 for CPT2. Immunolabeling was made visible with SuperSignal West Pico Plus detection solution (Thermo Fisher Scientific) in a G:Box ChemiXL1.4 (SynGene, Cambridge, United Kingdom). Densitometry analysis of target protein bands was performed with GeneTools (SynGene), and target protein expression was adjusted for any unequal loading and transfer by staining the membrane for protein using Amido Black.
Analysis of FAs
Lipids in cell lysates collected after different incubation times were extracted and separated into lipid classes according to the method previously described (30). Briefly, lipids were extracted with a methanol, water, and dichloromethane solution and fractionated in 2 aliquots to determine the FA composition in total cellular lipids and for separation into 4 lipid classes. CEs, TGs, NEFAs, and PLs were separated by solid-phase extraction on NH2 cartridges (Phenomenex, Torrance, CA, USA) (30), using hexane to elute CEs and 1% diethyl ether:10% dichloromethane in hexane to collect TGs. A solution of diethyl ether:acetic acid (95:5, v/v) (MilliporeSigma) was used to collect NEFAs, and finally methanol:chloroform (6:1, v/v) and 100% of methanol to elute the PLs. All the samples from the total lipid fraction and lipid classes were dried under nitrogen, resuspended in ethanol, and hydrolyzed with sodium hydroxide (1 N) at 90°C for 1 h. Free FAs were extracted with isooctane and derivatized to make pentafluorobenzyl FA esters prior to analysis by GC-MS in negative-ion chemical ionization mode as previously described (30, 33).
Quantification of labeled FAs in total cellular lipid fraction and lipid classes
Absolute FA concentrations of U[13C]-PA, U[13C]-OA, U[13C]-LA, and U[13C]-DHA as well as all unlabeled FAs in the same MW range were determined using calibration curves built with targeted internal standards. FA content was expressed in nanomoles of FA normalized to total cellular protein content, determined using a BCA assay (Pierce BCA Protein Assay Kit) (30). Uptake of each labeled FA by the PHT cells and their incorporation into the cellular lipid classes were measured at 2 and 30 h after U[13C]-FA incubation in PHT cells isolated from placentas of obese pregnant women and compared with those of normal BMI mothers.
In the total lipid fraction, we determined the presence of a [13C] label in other quantifiable FA species to assess whether PHTs are able to convert PA, OA, LA, or DHA into any other FA species over 36 h of incubation. The isotopic enrichment of PA and POA in the total lipid fraction was calculated using individually calibrated FA curves ranging from 0 to 100% of each U[13C]-FA enrichment relative to similar [12C]-FA enrichment and measured by GC-MS. Isotopic enrichments of PA and POA were expressed as mole percent excess (MPE) (%) over time (34). This unit expresses the molar ratio of tracer ([13C]) to the sum of the tracer ([13C]) + tracee ([12C]) extracted from the PHT cells. For example, the formula for determination of PA enrichment was: MPE (%) = [U[13C]-PA/(U[13C]-PA + [12C]-PA)] × 100.
Statistical analyses
Data are presented as means ± sem and means + sem. All statistical tests were performed using Prism v.6.07 (GraphPad Software, La Jolla, CA, USA). The Mann-Whitney U test was used to determine the statistical significance of differences between obese vs. control groups at 2 and 30 h. A value of P < 0.05 was considered significant.
RESULTS
PHT cells were cultured and differentiated for 66 h before incubation with uniformly stable isotope–labeled FAs (PA, OA, LA, DHA) complexed to FA-free BSA, and cells were subsequently collected at different times to determine the uptake of FA over time as well as the incorporation of different types of FA into cellular lipid fractions.
The absolute concentrations of labeled FA increase in total cellular lipid fraction over time
We determined the absolute concentrations of labeled FAs expressed in nanomoles of U[13C]-FA per microgram of protein for each FA in the total lipid fraction in PHT cells (Fig. 1A). Initially, the absolute concentrations of all FAs increased linearly over time, reflecting a rapid FA uptake and transport in these cells (Fig. 1A). As expected, the concentrations of U[13C]-FAs continued to increase over time, beyond 4 h, in the total lipid pool of cultured PHTs and reached a plateau between 24 and 36 h of incubation time (Fig. 1B), representing FA incorporation. Of the FAs added at the same concentration to culture medium (95 µM), PA and OA displayed similar levels over time, whereas LA incorporation was 2.5-fold less than PA or OA in PHT cells at equilibrium, reflecting lower levels of LA in the cells. U[13C]-DHA was present in the culture medium in a much lower concentration (15 µM) than the other FAs, reflecting physiologic FA concentration in the maternal circulation. DHA was rapidly taken up by the cells, and as expected, intracellular U[13C]-DHA concentrations remained lower that the other labeled FAs.
FAs are highly concentrated in PL fraction in PHT cells
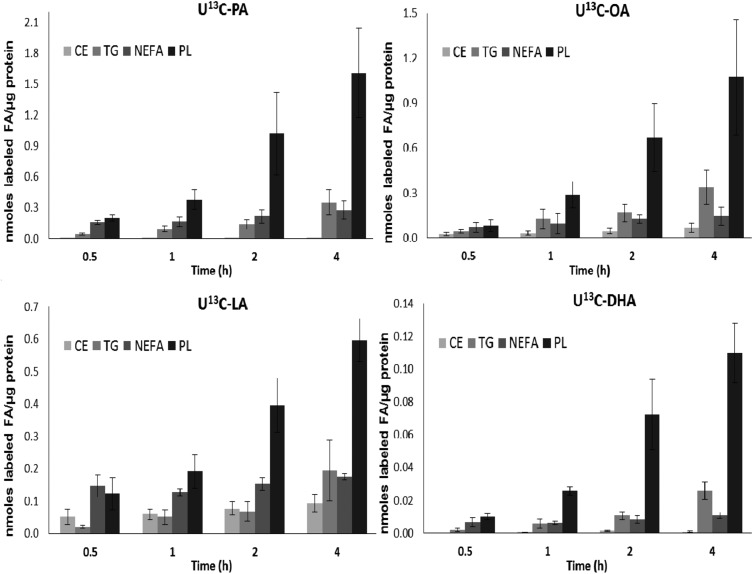
FAs taken up into cells are rapidly acted upon by coenzyme A synthase enzymes and shuttled to specific pools for either oxidation, re-esterification, or transport to the fetus. To provide novel insights on the mechanisms involved in the syncytiotrophoblast FA uptake and re-esterification, quantities of the labeled PA, OA, LA, and DHA were determined in 4 cellular lipid fractions over the initial 4 h of incubation (Fig. 2) and later times (Fig. 3). All 4 FAs were rapidly taken up by PHT cells and incorporated into the major lipid classes starting at 30 min of incubation. For all FAs studied, the highest concentrations were found in the PL fraction compared with other lipid fractions. This is indicative of a rapid synthesis and turnover of PLs and incorporation into PLs of NEFAs taken up from the maternal circulation.
Figure 2.
Uptake and incorporation of each labeled FA in lipid classes in cultured PHTs after the initial 4 h. Absolute concentrations of labeled PA, OA, LA, and DHA in 4 lipid classes in PHT cells isolated from normal-term placentas, expressed as nanomoles of FA per microgram of protein, were determined at 0.5, 1, 2, and 4 h after U[13C]-FA mix was added to culture medium (n = 3, means + sem).
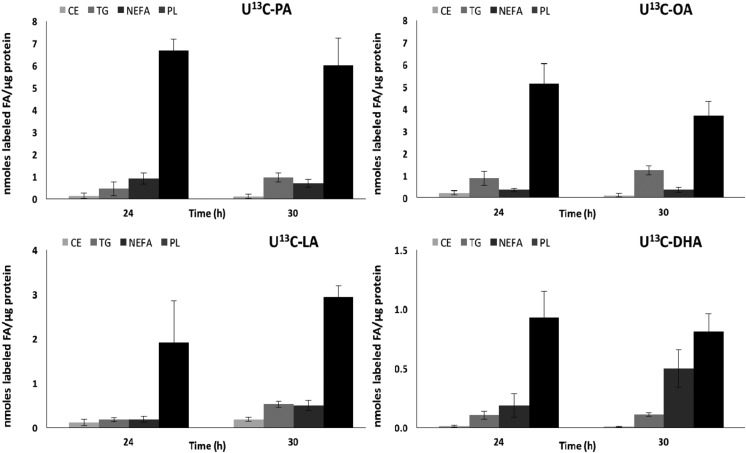
Figure 3.
Uptake and incorporation of each labeled FA in lipid classes in cultured PHTs after 24 and 30 h of incubation. Absolute concentrations of labeled PA, OA, LA, and DHA in 4 lipid classes in PHT cells isolated from normal-term placentas, expressed as nanomoles of FA per microgram of protein, were determined at 24 and 30 h to measure the FA esterification at the plateau (n = 3, means + sem).
Higher levels of unlabeled FAs in PHT cells isolated from obese mothers
Next, we determined the impact of maternal obesity on PHT FA uptake and metabolism using the same approach of incubation in uniformly stable isotope–labeled FAs (PA, OA, LA, DHA) complexed to FA-free BSA. PHT cells were collected at 2 h (uptake) and 30 h (incorporation), and the accumulation of [13C]-labeled FAs as well as the unlabeled FA pool were assessed.
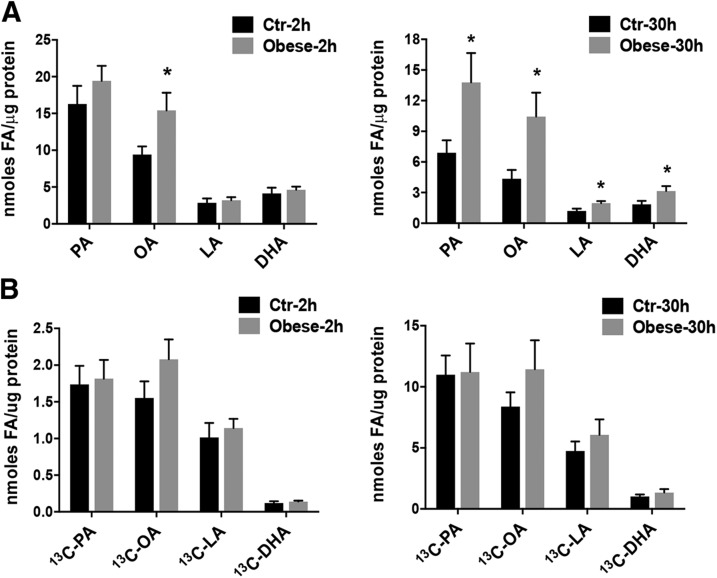
We found a significantly higher concentration of unlabeled SFAs, MUFAs, and essential FAs (n-6 and n-3 LCPUFA) in the total lipid pool in PHT cells isolated from obese women when compared with normal BMI placenta at 2 and 30 h of incubation (Fig. 4A). Specifically, after 2 h of incubation, the concentration of unlabeled OA was significantly higher in PHT cells isolated from obese mothers as compared with PHT cells from normal, healthy mothers. By 30 h of incubation, the unlabeled FA concentrations, although lower than at 2 h, were all significantly greater in PHT cells from obese pregnancies. These data suggest an alteration in FA metabolism and storage in PHTs from obese mothers that persist after 66–90 h of culture and when exposed to a physiologic mixture of FAs in the culture medium.
Figure 4.
The effect of maternal obesity on absolute concentrations of unlabeled FAs and on the uptake and incorporation of U[13C]-FAs in PHT cells. A) Absolute concentrations of unlabeled SFAs (PA), MUFAs (OA), and essential FAs (n-6 LCPUFA, LA, and n-3 LCPUFA, DHA) in PHT cells isolated from obese mothers (gray bars) compared with trophoblasts isolated from normal BMI mothers (Ctr; black bars) after incubation with U[13C]-FA mixture for 2 and 30 h. B) Uptake of U[13C]-FAs in PHT cells isolated from obese and normal BMI mothers after incubation with U[13C]-FA mixture for 2 and 30 h. Results are presented for the total cellular lipid pool and expressed as nanomoles of FA per microgram of protein for each labeled FA studied (n = 7 in each group, means + sem). *P < 0.05 (Mann-Whitney U test).
Maternal obesity does not modify the uptake of FAs in PHT cells
To determine whether the uptake of [13C]-labeled FAs in PHT cells isolated from pregnancies complicated by maternal obesity was different from normal-term pregnancies, we incubated PHT cells with our labeled FA mixture for 2 and 30 h and determined uptake into the total lipid pool. We found no difference in uptake of U[13C]-PA, U[13C]-OA, U[13C]-LA, and U[13C]-DHA in PHT cells isolated from obese mothers compared with placentas of normal BMI mothers (Fig. 4B).
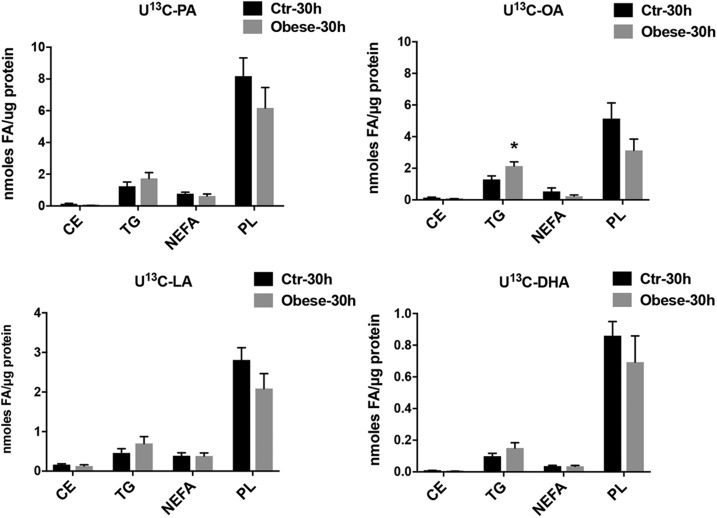
Maternal obesity alters FA esterification by increasing OA incorporation in TGs in PHT cells
To gain insight into cellular lipid metabolism and esterification in placentas of obese women, we quantified the labeled FAs in 4 main cellular lipid classes. After 30 h of incubation, the incorporation of [13C] PA, OA, and LA was not different in PL and CE fractions, whereas a significant increase was found in the OA content of the TG fraction (Fig. 5). These data suggest an accumulation of lipid in a storage form by the placental syncytiotrophoblast in pregnancies complicated by obesity.
Figure 5.
The effect of maternal obesity on the incorporation of U[13C]-FAs in lipid classes in PHT cells. FA esterification and incorporation into lipid fractions in PHT cells isolated from obese (gray bars) and normal BMI (Ctr; black bars) mothers at 30 h of incubation with U[13C]-FA mixture. Results are expressed as nanomoles of FA per microgram of protein for each labeled FA studied (n = 7 in each group, means + sem). *P < 0.05 (Mann-Whitney U test).
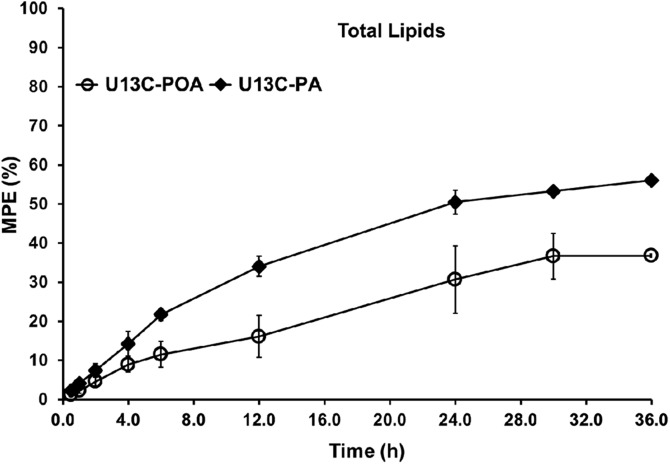
U[13C]-PA is converted into U[13C]-POA
Incubation with U[13C]-FAs allows for determination of conversion of labeled FAs to other FA species through the action of either elongase or desaturase enzymes. Figure 6 shows the conversion of U[13C]-PA (16:0) to U[13C]-POA (16:1 n-7) in total lipids in PHT cells over time. The conversion of PA to POA is catalyzed by SCD1; therefore, our data are consistent with the presence of an active Δ9 desaturase in PHT cells. The cellular enrichment of POA in total lipids over time is the result of the conversion of PA to POA and represents a lipid metabolic pathway not previously described in PHT cells. U[13C]-POA enrichments measured in total lipids mirror the cellular enrichment of PA over time (Fig. 6). Importantly, no other conversions of any of the other U[13C]-FAs were observed at any time because [13C] enrichments were not found in any other FA species in total lipids, suggesting there is no detectable elongase, Δ5, or Δ6 desaturase enzyme activities in PHT cells.
Figure 6.
Time course of cellular enrichment of POA (U[13C]-POA, MUFA) produced from the conversion of U[13C]-PA (SFA) added to the cultured medium in PHT cells isolated from normal BMI placentas. U[13C]-POA is expressed in mole percent excess (MPE, %) in the total lipids in the PHT cells over 36 h (n = 3 to 5 for each time, mean ± sem).
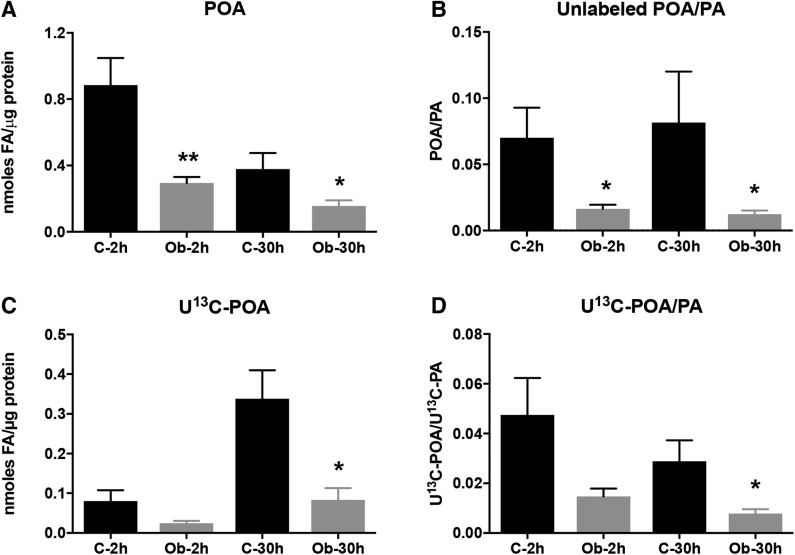
Decreased synthesis of POA and activity of SCD1 in PHT cells in maternal obesity
We found a marked decrease in endogenous unlabeled POA concentration in PHTs from pregnancies complicated by maternal obesity (Fig. 7), whereas unlabeled PA was unchanged at the 2 h time point (Fig. 4A). Decreased POA in PHTs isolated from obese mothers was also observed at 30 h in spite of a significantly increased concentration of unlabeled PA at this time point (Fig. 4A). Similarly, we found a decrease in the synthesis of U[13C]-POA from U[13C]-PA added to the medium at both 2 and 30 h of incubation in PHT cells isolated from placentas of obese mothers. These results are confirmed by the decreased ratio of U[13C]-POA to U[13C]-PA, reflecting a reduced SCD1 activity catalyzing the conversion of PA to POA (Fig. 7). These findings indicate a decreased placental syncytiotrophoblast POA synthesis in obese pregnant women, likely due to reduced SCD activity rather than limitations in substrate availability.
Figure 7.
Decreased synthesis of POA in PHT cells in maternal obesity. Unlabeled POA, newly synthesized U[13C]-POA, and the respective POA:PA ratios in total cellular lipid fraction in PHT cells isolated from obese (Ob, gray bars) and normal BMI (C, black bars) mothers at 2 and 30 h of incubation with U[13C]-FA mixture. A, B) Unlabeled POA concentration (A) and unlabeled POA:PA ratio (B) at 2 and 30 h in obese mothers vs. those with normal BMI. C) U[13C]-POA concentration produced from desaturation of U[13C]-PA at 2 and 30 h. D) U[13C]-POA:U[13C]-PA ratio in obese mothers vs. control group (n = 7 in each group, means + sem). *P < 0.05, **P < 0.01 (Mann-Whitney U test).
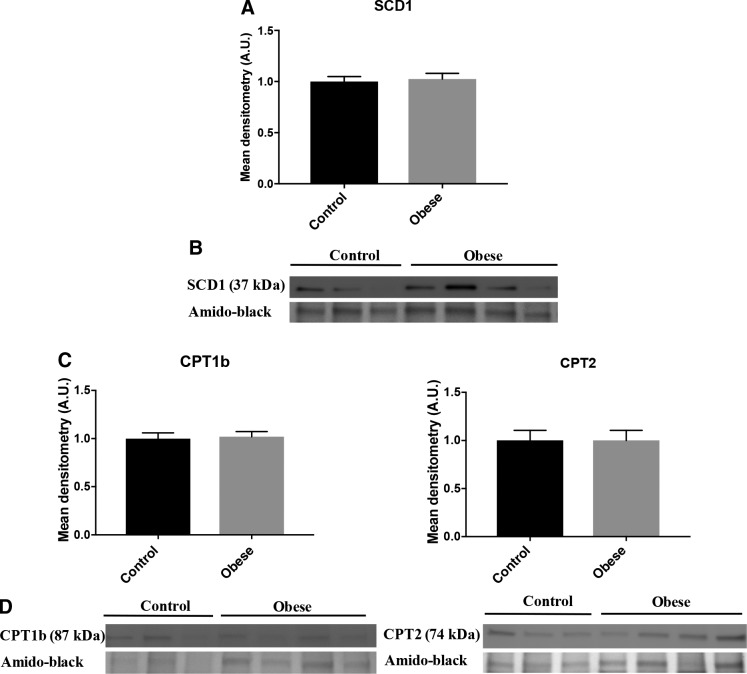
Maternal obesity does not alter protein expression of SCD1 in PHT cells
Using Western blot, we found no difference in protein expression of SCD1 in PHT cells in the obese vs. normal BMI group (Fig. 8A, B). The results are consistent with the fact that we found no change in the OA:SA ratio (unpublished results), which is also catalyzed by SCD1. These results are not entirely consistent with the decreased conversion of U[13C]-PA to U[13C]-POA, suggesting that other SCD isoforms may be involved in syncytiotrophoblast POA synthesis. However, we were not able to successfully identify SCD5 expression in PHTs, another isoform of SCD found exclusively in primates.
Figure 8.
Maternal obesity does not alter protein expression of SCD1, CPT1, or CPT2 in PHT cells. Effect of maternal obesity on lipogenesis and β-oxidation pathways in PHT cells. A, C) Relative protein expression of SCD1, CPT1b, and CPT2 in PHT cells isolated from obese (gray bars) and normal BMI (black bars) mothers. B, D) Representative Western blot of SCD1, CPT1b, and CPT2 (n = 7 in each group, means + sem); P < 0.05 was considered significant (Mann-Whitney U test). A.U., arbitrary unit.
Maternal obesity does not affect the β-oxidation pathway in PHT cells
Increased cellular TG synthesis is believed to be associated with a decrease in the β-oxidation pathway. We measured protein expression of CPT1b and CPT2, 2 enzymes representing the rate-limiting synthesis of the β-oxidation, in PHT cells from obese and normal BMI mothers. We found no significant differences in the protein expression of these 2 specific enzymes in PHT cells (Fig. 8C, D).
DISCUSSION
We developed a novel approach utilizing 4 stable isotope–labeled FA species to study the uptake and incorporation of FAs in a cultured primary human syncytiotrophoblast, a well-established and physiologically relevant model for the study of human placental function. In normal pregnancies, all 4 of the U[13C]-FAs were rapidly taken up and differentially incorporated into the total cellular lipid pool in PHT cells over a 36-h time course. Moreover, the syncytiotrophoblast displayed a rapid uptake and incorporation of all 4 U[13C]-FAs studied into the PL fraction, reflecting a strong preference for this cellular lipid fraction in this transporting epithelium. PLs are critical substrates for plasma membrane production, and we recently suggested that PL intermediaries, in particular lysophosphatidylcholine, may be transferred to the fetus across the basal membrane via major facilitator superfamily domain containing 2a (Mfsd2a), a specific lysophosphatidylcholine transporter (35). In addition, we report for the first time the synthesis of U[13C]-POA, a lipid signaling molecule with insulin-sensitizing and anti-inflammatory properties, resulting from conversion of U[13C]-PA by SCD activity. Importantly, POA synthesis was decreased in PHT cells from obese pregnancies, which may contribute to insulin resistance and low-grade inflammation in obese pregnant women.
In this study, 4 stable isotope–labeled [13C] FA species—PA (16:0), OA (18:1 n-9), LA (18:2 n-6), and DHA (22:6 n-3)—representing an SFA, a MUFA, an essential polyunsaturated FA, and an n-3 LCPUFA were added to culture medium in physiologic concentrations to establish a novel methodology for studying FA uptake, esterification, and metabolism by the primary human syncytiotrophoblast. In PHTs from healthy pregnancy, U[13C]-FA levels increased rapidly over time, reaching a plateau at about 24 h. The data also show that PHT cells prioritize the de novo synthesis and remodeling of PLs over other cellular lipid forms, consistent with our previous study (30). These findings are also in agreement with the study of Perazzolo et al. (36) using perfused human cotyledons and with the in vivo results reported by Gil-Sánchez et al. (37) showing a higher U[13C]-FA concentration in placental PL than in any other lipid fractions when labeled PA, OA, LA, and DHA were orally administered to pregnant women 12 h before delivery. The rapid incorporation of the FAs into more complex forms suggests that PHTs are converting these FAs into less toxic forms for cellular storage and later utilization. However, the mechanisms by which FAs are released from intracellular esterified lipid pools for transfer across the syncytiotrophoblast basal plasma membrane and delivery to the fetus remain largely unknown.
In the total cellular lipid pool, U[13C]-OA and U[13C]-PA are the FAs in greatest abundance, reflective of their high levels in the incubation medium and in maternal circulation. These 2 FAs had the greatest overall uptake by PHT cells over time and were incorporated preferentially into PLs and then TGs followed by CEs; only low levels remained as NEFAs in PHT cells. OA is the major MUFA in maternal circulation and is found in very high levels in membrane PLs of red blood cells at term (38) as well as being abundantly incorporated into phosphatidylcholine in placental tissue (39). PA is also present in high abundance in maternal circulation and is considered lipotoxic and atherogenic in other tissues (40). Although the uptake and incorporation of DHA appears to be slower than that for the other 3 FAs, in particular when compared with U[13C]-LA (an essential LCPUFA), it is important to consider the differences in extracellular concentrations of the FAs to fully understand the handling of DHA. We aimed to represent physiologic U[13C]-DHA concentrations and used 15 µM in the medium, whereas the other 3 were used at 95 µM, in accordance with relative circulating levels in late-term pregnant women. Considering the rapid uptake of DHA from the low extracellular levels, preferential handling in uptake and incorporation of DHA by the syncytiotrophoblast, as previously reported by Gil-Sánchez et al. (37), is likely occurring when the FAs are presented together, but because of low availability of DHA, the intracellular levels remain low.
In PHT cells isolated from the placenta of obese mothers, we found higher concentrations of unlabeled PA, OA, LA and DHA, whereas the uptake of U[13C]-PA, U[13C]-OA, U[13C]-LA, and U[13C]-DHA into the total lipid pool was not different at 2 and 30 h compared with PHTs from mothers with normal BMI. However, we observed a significant increase of U[13C]-OA in the TG fraction after 30 h of incubation, which is consistent with a high cellular abundance and the major role of OA to promote the TG synthesis (41). These findings are in agreement with previous studies showing higher lipid levels in the total lipid fraction and storage in cellular TG pools in the placenta of obese pregnant women (42–44). The current study extends these observations by identifying OA as the predominant FA accumulating in placental TG in maternal obesity.
Increased placental lipid storage has been associated with a decrease in FA oxidation capacity in trophoblast cells isolated from normal BMI placentas when incubated with a high concentration of PA and OA (45). The transport of FAs across the mitochondrial membrane is mediated by CPT1 and CPT2 (46, 47), which represents the rate-limiting step in mitochondrial β-oxidation of long-chain FAs. Although we did not measure FA oxidation directly, the protein expression of CPT1b and CPT2 in PHT cells was not affected by maternal obesity in our study. These data suggest that the mitochondrial FA oxidation capacity remains intact. Our findings are consistent with the study of Calabuig-Navarro et al. (42) showing no changes in the placental protein expression of CPT1 as well as no change in the rate of [3H]palmitate oxidation in PHT cells isolated from pregnancies complicated by maternal obesity. Our findings are not in complete agreement with the report by Pathmaperuma et al. (45) in which they demonstrated a decreased β-oxidation in human trophoblast cells isolated from normal-term placentas treated with a PA:OA (1:1) mixture to mimic hyperlipidemia. One key difference that may explain the different findings in the 2 studies is the concentrations of FAs used. We used much lower concentrations of PA and OA (0.095 mM), reflecting physiologic levels of FA in maternal circulation compared with the 0.25 mM PA and OA mixture used in the study by Pathmaperuma et al. (45). In addition, using high concentrations of only 2 FA species in cell culture conditions may not fully represent in vivo hyperlipidemia of pregnancy.
In our model using physiologic U[13C]-LA concentrations, we found no detectable [13C] label in any n-6 LCPUFA, including arachidonic acid species (20:4 n-6), therefore no arachidonic acid was formed from U[13C]-LA in the total cellular lipid fraction over the 30 h of incubation. Likewise, we found no evidence of conversion of U[13C]-PA (16:0) into SA (18:0). These findings confirm a very low or absent activity of Δ6 and Δ5 desaturases and elongases in PHT cells and are in agreement with a previous report by Hanebutt et al. (48) of low or nonexistent activity of these enzymes in placental tissue.
The levels of POA in the diet are low, suggesting that POA is largely produced by endogenous desaturation of the most abundant SFA, PA, by SCD. Interestingly, we measured an isotopic enrichment of POA derived from U[13C]-PA conversion, demonstrating an activity of SCD in PHT cells. POA has been called a lipokine and reported to act as an insulin sensitizer that improves glucose metabolism (20) and has anti-inflammatory properties (22), and it is therefore possible that placental POA synthesis may modulate placental, fetal, or maternal insulin sensitivity and inflammation (or a combination of all of these).
We report that POA:PA and U[13C]-POA:U[13C]-PA ratios are decreased in PHT cells isolated from obese mothers, reflecting the decreased conversion of PA to POA. We did not find changes in SCD1 protein expression in PHT cells isolated from pregnancies complicated by maternal obesity, which is in contrast to a recent study by Calabuig-Navarro et al. (42) reporting an increased placental SCD1 protein expression in obese women. We speculate that the activity of SCD1 in the syncytiotrophoblast is regulated by post-translational modification in PHTs. Because POA is a known insulin sensitizer and anti-inflammatory lipokine (49), decreased syncytiotrophoblast POA synthesis may contribute to insulin resistance and low-grade inflammation in obese pregnant women.
In summary, PHT cells take up FAs rapidly and preferentially use them to synthesize PLs. In trophoblast cells isolated from obese mothers, we found a continued accumulation of unlabeled FAs in PHTs after 30 h of labeled FA incubation and an increase in the esterification and storage of OA in the TG fraction but no indication of alterations of the FA oxidation capacity. We found activity of SCD, a desaturase enzyme that converts PA to POA, an anti-inflammatory and insulin-sensitizing signaling lipid. However, no other desaturase or elongase activity was found. Although little is known about the exact role of POA in syncytiotrophoblast cells, it may have a functional role in the placenta and beyond to modulate inflammation and insulin sensitivity. We speculate that decreased syncytiotrophoblast POA synthesis may contribute to insulin resistance and low-grade inflammation in obese pregnant women.
ACKNOWLEDGMENTS
Placental collection was supported by the Perinatal Clinical Translational Research Center at the University of Colorado Anschutz Medical Campus. This work was supported by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grant DK048520-23, and NIH National Center for Advancing Translational Sciences Colorado Clinical and Translational Sciences Institute Clinical and Translational Science Award Grant UL1 TR002535. The authors declare no conflicts of interest.
Glossary
- BCA
bicinchoninic acid
- BMI
body mass index
- BSA
bovine serum albumin
- CE
cholesterol ester
- CPT
carnitine palmitoyltransferase
- DHA
docosahexaenoic acid
- FA
fatty acid
- hCG
human chorionic gonadotropin
- LA
linoleic acid
- LCPUFA
long-chain polyunsaturated FA
- MPE
mole percent excess
- MUFA
monounsaturated FA
- NEFA
nonesterified FA
- OA
oleic acid
- PA
palmitic acid
- PHT
primary human trophoblast
- PL
phospholipid
- POA
palmitoleic acid
- SA
stearic acid
- SCD
stearoyl–coenzyme A desaturase
- SFA
saturated FA
- TBS
Tris-buffered saline
- TG
triglyceride
- U[13C]
uniformly labeled [13C]
AUTHOR CONTRIBUTIONS
V. Ferchaud-Roucher, T. Jansson, and T. L. Powell designed the research studies; V. Ferchaud-Roucher conducted the experiments, analyzed the results, and wrote the manuscript; K. Barner performed the Western blot experiments; and all authors discussed the results and edited the manuscript.
REFERENCES
- 1.Ogden C. L., Carroll M. D., Kit B. K., Flegal K. M. (2014) Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311, 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher S. C., Kim S. Y., Sharma A. J., Rochat R., Morrow B. (2013) Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003-2009. Prev. Med. 56, 372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano P. M., Ehrenberg H. M. (2006) The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 113, 1126–1133 [DOI] [PubMed] [Google Scholar]
- 4.Baeten J. M., Bukusi E. A., Lambe M. (2001) Pregnancy complications and outcomes among overweight and obese nulliparous women. Am. J. Public Health 91, 436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebire N. J., Jolly M., Harris J. P., Wadsworth J., Joffe M., Beard R. W., Regan L., Robinson S. (2001) Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int. J. Obes. Relat. Metab. Disord. 25, 1175–1182 [DOI] [PubMed] [Google Scholar]
- 6.Sewell M. F., Huston-Presley L., Super D. M., Catalano P. (2006) Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am. J. Obstet. Gynecol. 195, 1100–1103 [DOI] [PubMed] [Google Scholar]
- 7.Catalano P. M., Presley L., Minium J., Hauguel-de Mouzon S. (2009) Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32, 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochner H., Friedlander Y., Calderon-Margalit R., Meiner V., Sagy Y., Avgil-Tsadok M., Burger A., Savitsky B., Siscovick D. S., Manor O. (2012) Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 125, 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons T. J., Power C., Manor O. (2001) Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ 323, 1331–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boney C. M., Verma A., Tucker R., Vohr B. R. (2005) Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115, e290–e296 [DOI] [PubMed] [Google Scholar]
- 11.Herrera E., Amusquivar E., López-Soldado I., Ortega H. (2006) Maternal lipid metabolism and placental lipid transfer. Horm. Res. 65 (Suppl 3), 59–64 [DOI] [PubMed] [Google Scholar]
- 12.Jarvie E., Hauguel-de-Mouzon S., Nelson S. M., Sattar N., Catalano P. M., Freeman D. J. (2010) Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin. Sci. (Lond.) 119, 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindegaard M. L., Olivecrona G., Christoffersen C., Kratky D., Hannibal J., Petersen B. L., Zechner R., Damm P., Nielsen L. B. (2005) Endothelial and lipoprotein lipases in human and mouse placenta. J. Lipid Res. 46, 2339–2346 [DOI] [PubMed] [Google Scholar]
- 14.Dube E., Gravel A., Martin C., Desparois G., Moussa I., Ethier-Chiasson M., Forest J. C., Giguère Y., Masse A., Lafond J., et al. 2012) Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol. Reprod. 87, 14, 1–11. [DOI] [PubMed] [Google Scholar]
- 15.Brass E., Hanson E., O’Tierney-Ginn P. F. (2013) Placental oleic acid uptake is lower in male offspring of obese women. Placenta 34, 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassance L., Haghiac M., Leahy P., Basu S., Minium J., Zhou J., Reider M., Catalano P. M., Hauguel-de Mouzon S., et al. 2015) Identification of early transcriptome signatures in placenta exposed to insulin and obesity. Am. J. Obstet. Gynecol. 212, 647.e1–647.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ntambi J. M., Miyazaki M., Stoehr J. P., Lan H., Kendziorski C. M., Yandell B. S., Song Y., Cohen P., Friedman J. M., Attie A. D. (2002) Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 99, 11482–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vázquez-Vela M. E., Torres N., Tovar A. R. (2008) White adipose tissue as endocrine organ and its role in obesity. Arch. Med. Res. 39, 715–728 [DOI] [PubMed] [Google Scholar]
- 19.Flowers J. B., Rabaglia M. E., Schueler K. L., Flowers M. T., Lan H., Keller M. P., Ntambi J. M., Attie A. D. (2007) Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes 56, 1228–1239 [DOI] [PubMed] [Google Scholar]
- 20.Cao H., Gerhold K., Mayers J. R., Wiest M. M., Watkins S. M., Hotamisligil G. S. (2008) Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frigolet M. E., Gutiérrez-Aguilar R. (2017) The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr. 8, 173S–181S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan K. L., Pillon N. J., Sivaloganathan D. M., Costford S. R., Liu Z., Théret M., Chazaud B., Klip A. (2015) Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J. Biol. Chem. 290, 16979–16988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talbot N. A., Wheeler-Jones C. P., Cleasby M. E. (2014) Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol. Cell. Endocrinol. 393, 129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risérus U., Tan G. D., Fielding B. A., Neville M. J., Currie J., Savage D. B., Chatterjee V. K., Frayn K. N., O’Rahilly S., Karpe F. (2005) Rosiglitazone increases indexes of stearoyl-CoA desaturase activity in humans: link to insulin sensitization and the role of dominant-negative mutation in peroxisome proliferator-activated receptor-gamma. Diabetes 54, 1379–1384 [DOI] [PubMed] [Google Scholar]
- 25.Lager S., Jansson N., Olsson A. L., Wennergren M., Jansson T., Powell T. L. (2011) Effect of IL-6 and TNF-α on fatty acid uptake in cultured human primary trophoblast cells. Placenta 32, 121–127 [DOI] [PubMed] [Google Scholar]
- 26.Lager S., Gaccioli F., Ramirez V. I., Jones H. N., Jansson T., Powell T. L. (2013) Oleic acid stimulates system A amino acid transport in primary human trophoblast cells mediated by toll-like receptor 4. J. Lipid Res. 54, 725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aye I. L., Gao X., Weintraub S. T., Jansson T., Powell T. L. (2014) Adiponectin inhibits insulin function in primary trophoblasts by PPARα-mediated ceramide synthesis. Mol. Endocrinol. 28, 512–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones H. N., Jansson T., Powell T. L. (2010) Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino Acid transport in human primary trophoblast cells. Diabetes 59, 1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosario F. J., Kanai Y., Powell T. L., Jansson T. (2013) Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J. Physiol. 591, 609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferchaud-Roucher V., Rudolph M. C., Jansson T., Powell T. L. (2017) Fatty acid and lipid profiles in primary human trophoblast over 90h in culture. Prostaglandins Leukot. Essent. Fatty Acids 121, 14–20 [DOI] [PubMed] [Google Scholar]
- 31.Lager S., Ramirez V. I., Gaccioli F., Jansson T., Powell T. L. (2014) Expression and localization of the omega-3 fatty acid receptor GPR120 in human term placenta. Placenta 35, 523–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lager S., Ramirez V. I., Gaccioli F., Jang B., Jansson T., Powell T. L. (2016) Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta 40, 60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph M. C., Karl Maluf N., Wellberg E. A., Johnson C. A., Murphy R. C., Anderson S. M. (2012) Mammalian fatty acid synthase activity from crude tissue lysates tracing 13C-labeled substrates using gas chromatography-mass spectrometry. Anal. Biochem. 428, 158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cobelli C., Toffolo G., Foster D. M. (1992) Tracer-to-tracee ratio for analysis of stable isotope tracer data: link with radioactive kinetic formalism. Am. J. Physiol. 262, E968–E975 [DOI] [PubMed] [Google Scholar]
- 35.Ferchaud-Roucher V., Kramer A., Silva E., Pantham P., Weintraub S. T., Jansson T., Powell T. L. (2019) A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids. 1864, 394–402 [DOI] [PubMed] [Google Scholar]
- 36.Perazzolo S., Hirschmugl B., Wadsack C., Desoye G., Lewis R. M., Sengers B. G. (2017) The influence of placental metabolism on fatty acid transfer to the fetus. J. Lipid Res. 58, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil-Sánchez A., Larqué E., Demmelmair H., Acien M. I., Faber F. L., Parrilla J. J., Koletzko B. (2010) Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am. J. Clin. Nutr. 92, 115–122 [DOI] [PubMed] [Google Scholar]
- 38.Min Y., Ghebremeskel K., Lowy C., Thomas B., Crawford M. A. (2004) Adverse effect of obesity on red cell membrane arachidonic and docosahexaenoic acids in gestational diabetes. Diabetologia 47, 75–81 [DOI] [PubMed] [Google Scholar]
- 39.Uhl O., Demmelmair H., Segura M. T., Florido J., Rueda R., Campoy C., Koletzko B. (2015) Effects of obesity and gestational diabetes mellitus on placental phospholipids. Diabetes Res. Clin. Pract. 109, 364–371 [DOI] [PubMed] [Google Scholar]
- 40.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., Hotamisligil G. S. (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391; erratum: 16, 237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusunoki J., Kanatani A., Moller D. E. (2006) Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine 29, 91–100 [DOI] [PubMed] [Google Scholar]
- 42.Calabuig-Navarro V., Haghiac M., Minium J., Glazebrook P., Ranasinghe G. C., Hoppel C., Hauguel de-Mouzon S., Catalano P., O’Tierney-Ginn P. (2017) Effect of maternal obesity on placental lipid metabolism. Endocrinology 158, 2543–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saben J., Lindsey F., Zhong Y., Thakali K., Badger T. M., Andres A., Gomez-Acevedo H., Shankar K. (2014) Maternal obesity is associated with a lipotoxic placental environment. Placenta 35, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschmugl B., Desoye G., Catalano P., Klymiuk I., Scharnagl H., Payr S., Kitzinger E., Schliefsteiner C., Lang U., Wadsack C., Hauguel-de Mouzon S. (2017) Maternal obesity modulates intracellular lipid turnover in the human term placenta. Int. J. Obes. 41, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathmaperuma A. N., Maña P., Cheung S. N., Kugathas K., Josiah A., Koina M. E., Broomfield A., Delghingaro-Augusto V., Ellwood D. A., Dahlstrom J. E., Nolan C. J. (2010) Fatty acids alter glycerolipid metabolism and induce lipid droplet formation, syncytialisation and cytokine production in human trophoblasts with minimal glucose effect or interaction. Placenta 31, 230–239 [DOI] [PubMed] [Google Scholar]
- 46.Sharma S., Black S. M. (2009) Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov. Today Dis. Mech. 6, e31–e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flanagan J. L., Simmons P. A., Vehige J., Willcox M. D., Garrett Q. (2010) Role of carnitine in disease. Nutr. Metab. (Lond.) 7, 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanebutt F. L., Demmelmair H., Schiessl B., Larqué E., Koletzko B. (2008) Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin. Nutr. 27, 685–693 [DOI] [PubMed] [Google Scholar]
- 49.De Souza C. O., Valenzuela C. A., Baker E. J., Miles E. A., Rosa Neto J. C., Calder P. C. (2018) Palmitoleic acid has stronger anti-inflammatory potential in human endothelial cells compared to oleic and palmitic acids. Mol. Nutr. Food Res. 62, e1800322 [DOI] [PubMed] [Google Scholar]