7.
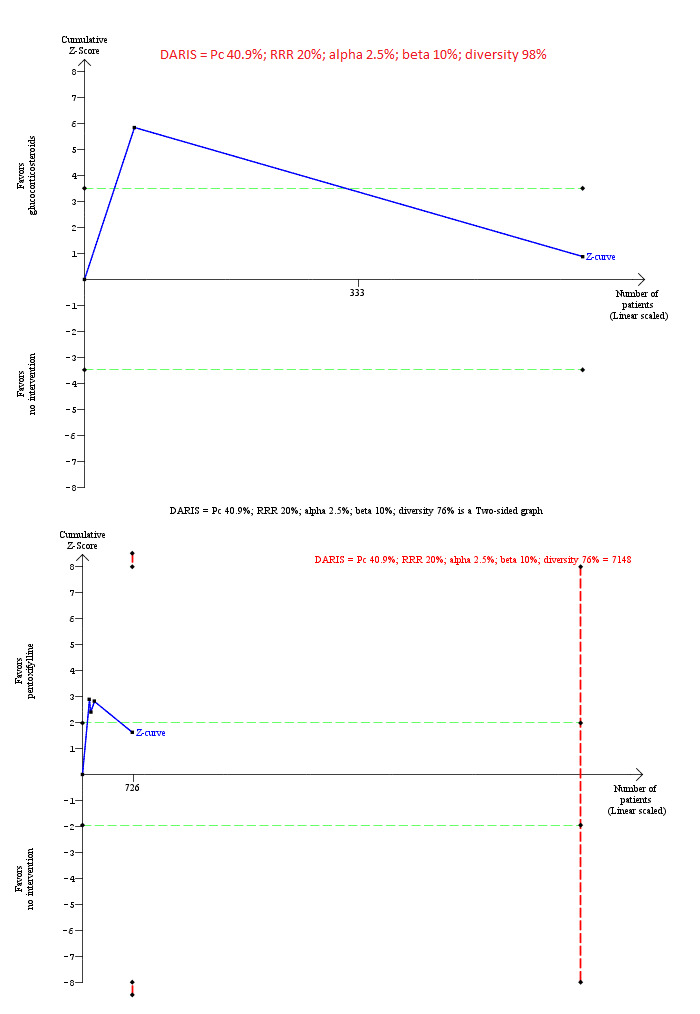
Trial Sequential Analysis of mortality at maximal follow‐up (severe alcoholic hepatitis): glucocorticosteroids versus no intervention and pentoxifylline versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 40.9%), and diversity observed in the analyses (98% and 76%), the accrued sample size (607 participants for glucocorticosteroids versus no intervention and 726 participants for pentoxifylline versus no intervention) was lower than the diversity‐adjusted required information size (DARIS) (72,755 participants for glucocorticosteroids versus no intervention and 7148 participants for pentoxifylline versus no intervention). For glucocorticosteroids versus no intervention, the accrued sample size was so small that the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) crosses the conventional boundaries (dotted green lines) favouring glucocorticosteroids and pentoxifylline for the two comparisons, but does not cross the conventional boundaries after the large trial (Thursz 2015). The Z‐curve does not cross any of trial sequential monitoring boundaries (dotted red lines) for pentoxifylline versus no intervention. This indicates that there is a high risk of random errors in both these comparisons.
