Figure 2. Model of EBI2-mediated B and T cell co-localization.
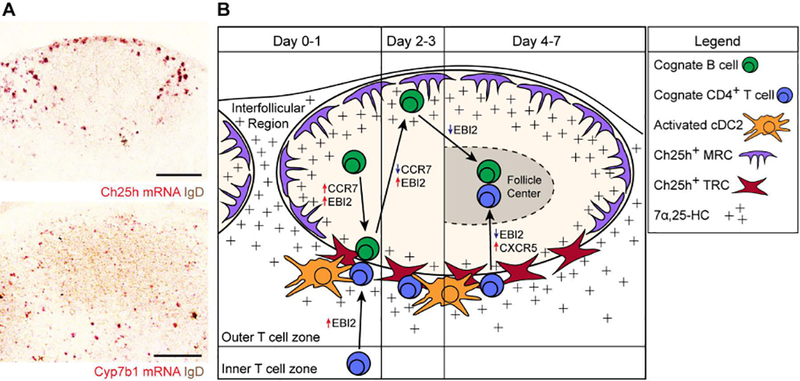
(A) RNAscope in situ hybridization for Ch25h and Cyp7b1 mRNA expression (red signal) relative to endogenous B cells (IgD, brown) in lymph node sections. Ch25h signal is enriched in outer- and inter-follicular regions and at the follicle-T zone interface. Cyp7b1 signal is also detected in these regions. (B) Model for cognate B cell and CD4+ T cell movement after immunization. Early after immunization (Day 0–1), B cells upregulate EBI2 and CCR7 to promote positioning along the follicle-T zone interface. Concurrently, cognate CD4+ T cells also upregulate EBI2 to position at this interface, where they interact with activated cDC2s as well as the activated B cells. A subset of T zone fibroblastic reticular cells (TRCs) expresses Ch25h, supporting the 7α,25-HC gradient that guides cells to this location. During days 2–3, B cells downregulate CCR7 and maintain upregulated EBI2 expression, promoting their positioning at outer-follicular and inter-follicular locations. In these locations, Ch25h-expressing marginal reticular cells (MRCs) support the 7α,25-HC gradient. The cognate CD4+ T cells continue to receive signals from activated cDC2s to promote their differentiation into T follicular helper (Tfh) cells. During days 4–7, the cognate B cells downregulate EBI2 to promote their positioning at the center of the follicle to form GCs. The cognate CD4+ T cells that have developed into Tfh cells utilize CXCR5 to move into the follicle, where they support the GC response. These cells have been shown to express lower levels of EBI2, supporting their positioning in GCs (144).
