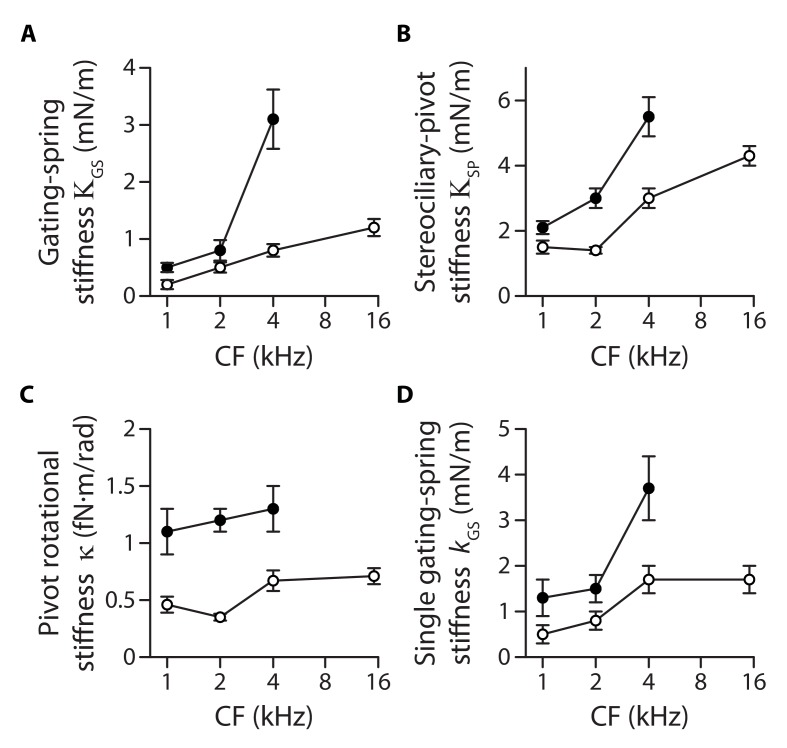
Figure 3. Stiffness gradients of the gating springs and of the stereociliary pivots.
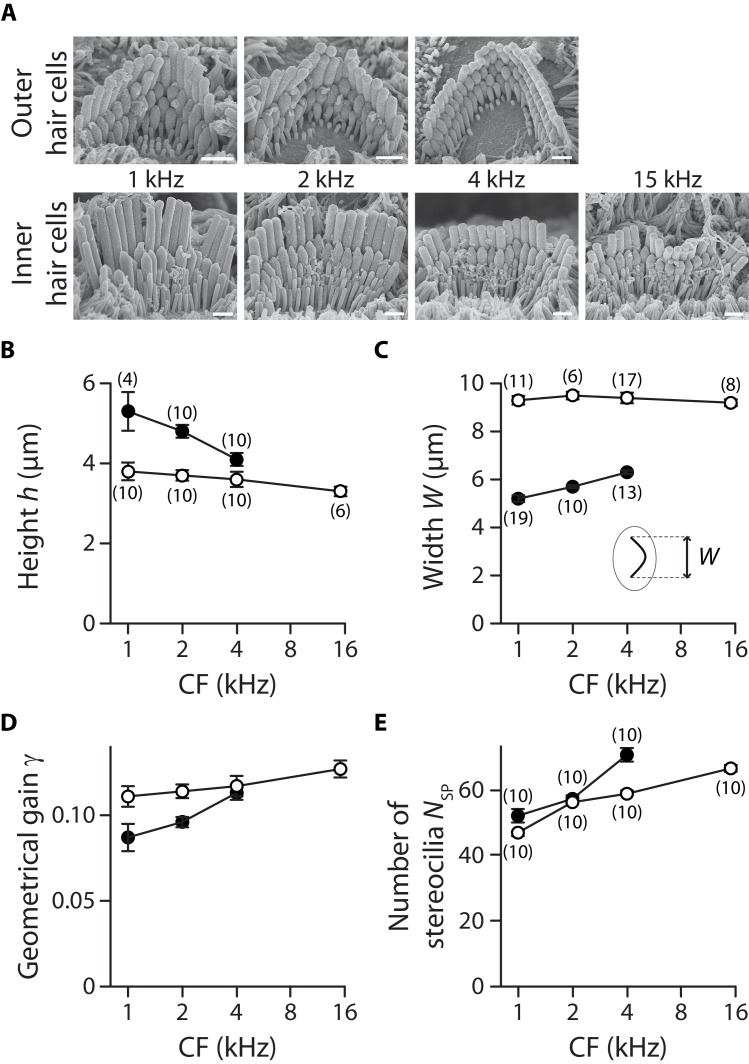
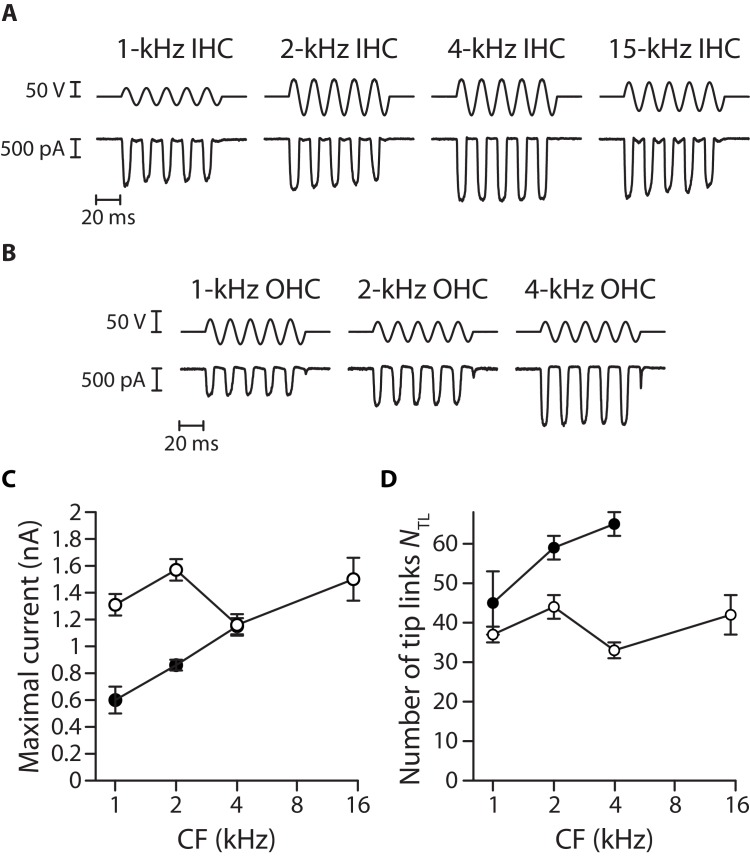
Stiffness (A) of the contribution of the gating springs (), (B) of a hair bundle after tip-link disruption, corresponding to the contribution of the stereociliary pivots (), (C) of a single stereociliary pivot (), and (D) of a single gating spring () as a function of the characteristic frequency (CF) for inner (white disks) and outer (black disks) hair cells. These stiffnesses were calculated from measured values of the hair-bundle stiffness (Figure 2), the amplitude ratio of hair-bundle movements before and after tip-link disruption (Figure 3—figure supplement 1), the hair-bundle height and the number of stereocilia (Figure 3—figure supplement 2), and the average number of intact tip links (Figure 3—figure supplement 3). Each data point is the mean ± SEM; SEMs were calculated as described in the Materials and methods.
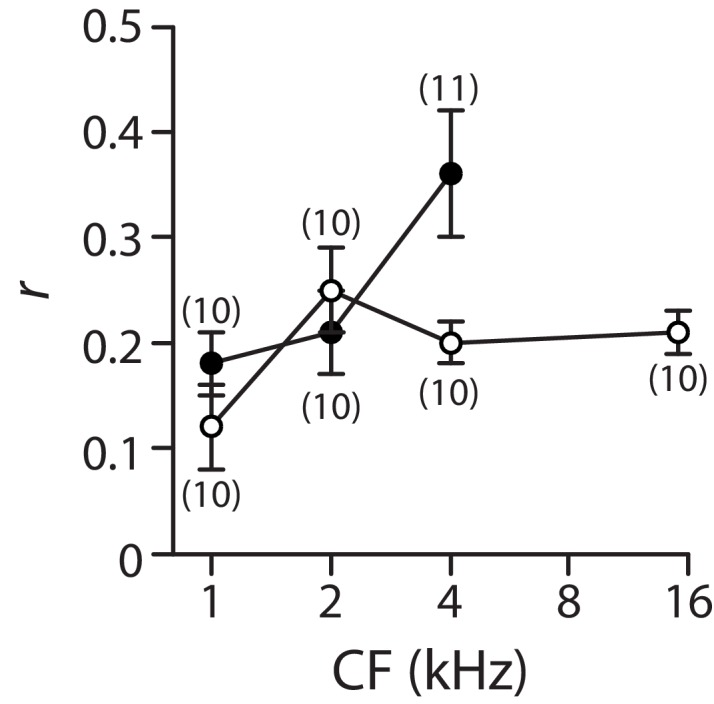
Figure 3—figure supplement 1. Gating-spring contribution to the hair-bundle stiffness.