5.
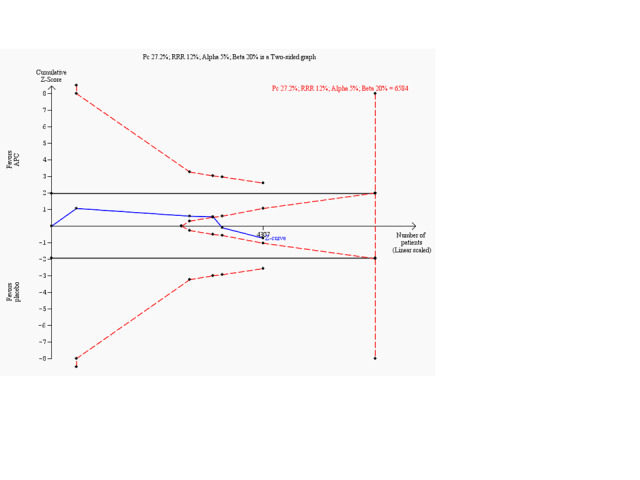
Trial sequential analysis of human recombinant activated protein C (APC) versus placebo in‐hospital mortality based on the diversity‐adjusted required information size (DARIS) of 6584 patients. This DARIS was calculated based upon a proportion of patients dying in‐hospital out of 27.2% in the control group; a RRR of 12% in the experimental intervention group; an alpha (α) of 5%; a beta (β) of 20%; and a diversity of 15%. The cumulative Z‐curve (blue line) crossed the trial sequential beta‐spending monitoring boundary, showing that the area of futility has been reached. This suggests that no more trials may be needed for disproving an intervention effect of 12% relative risk reduction. Smaller risk reductions might still require further trials.
