Abstract
Background
Contracts are a verbal or written agreement that a patient makes with themselves, with healthcare practitioners, or with carers, where participants commit to a set of behaviours related to the care of a patient. Contracts aim to improve the patients' adherence to treatment or health promotion programmes.
Objectives
To assess the effects of contracts between patients and healthcare practitioners on patients' adherence to treatment, prevention and health promotion activities, the stated health or behaviour aims in the contract, patient satisfaction or other relevant outcomes, including health practitioner behaviour and views, health status, reported harms, costs, or denial of treatment as a result of the contract.
Search methods
We searched: the Cochrane Consumers and Communication Review Group's Specialised Register (in May 2004); the Cochrane Central Register of Controlled Trials (CENTRAL), (The Cochrane Library 2004, issue 1); MEDLINE 1966 to May 2004); EMBASE (1980 to May 2004); PsycINFO (1966 to May 2004); CINAHL (1982 to May 2004); Dissertation Abstracts. A: Humanities and Social Sciences (1966 to May 2004); Sociological Abstracts (1963 to May 2004); UK National Research Register (2000 to May 2004); and C2‐SPECTR, Campbell Collaboration (1950 to May 2004).
Selection criteria
We included randomised controlled trials comparing the effects of contracts between healthcare practitioners and patients or their carers on patient adherence, applied to diagnostic procedures, therapeutic regimens or any health promotion or illness prevention initiative for patients. Contracts had to specify at least one activity to be observed and a commitment of adherence to it. We included trials comparing contracts with routine care or any other intervention.
Data collection and analysis
Selection and quality assessment of trials were conducted independently by two review authors; single data extraction was checked by a statistician. We present the data as a narrative summary, given the wide range of interventions, participants, settings and outcomes, grouped by the health problem being addressed.
Main results
We included thirty trials, all conducted in high income countries, involving 4691 participants. Median sample size per group was 21. We examined the quality of each trial against eight standard criteria, and all trials were inadequate in relation to three or more of these standards. Trials evaluated contracts in addiction (10 trials), hypertension (4 trials), weight control (3 trials) and a variety of other areas (13 trials). Fifteen trials reported at least one outcome that showed statistically significant differences favouring the contracts group, six trials reported at least one outcome that showed differences favouring the control group and 26 trials reported at least one outcome without differences between groups. Effects on adherence were not detected when measured over longer periods.
Authors' conclusions
There is limited evidence that contracts can potentially contribute to improving adherence, but there is insufficient evidence from large, good quality studies to routinely recommend contracts for improving adherence to treatment or preventive health regimens.
Keywords: Humans, Patient Compliance, Physician‐Patient Relations, Community Participation, Contracts, Contracts/standards, Health Promotion, Health Promotion/methods, Randomized Controlled Trials as Topic
Contracts between patients and healthcare practitioners for improving patients' adherence to recommended healthcare activities
Sometimes patients do not complete a course of treatment or they do not follow recommended changes in diet or personal habits. This poor adherence may be because treatments take a long time, have side effects or involve changing patients' habits, which is often difficult. Several interventions aim to change the relationship between patients and healthcare practitioners in order to improve the patients' adherence to treatments. One of these interventions is in the form of contracts between healthcare practitioners and patients, by which one or both parties commit to a set of behaviours related to the care of the patient. Contracts may be written or verbal. Most contracts are between healthcare practitioners and patients, but they may also occur between practitioners and carers, carers and patients or by a patient with him/herself. In this review we assessed whether contracts between practitioners and patients really improve the patients' adherence to treatment or their health status. We also assessed the effects of contracts on other outcomes, including patient participation and satisfaction, health practitioner behaviour and views, health status, harms, costs, and ethical issues.
We found 30 trials involving 4691 participants, examining several types of contracts. The main health problems targeted were substance addictions, hypertension and overweight. Many of the trials were of poor quality and involved small numbers of people. Most were conducted in the USA. In 15 of the trials there was at least one outcome showing statistically significant differences in favour of the contracts group (although some of the improvements in adherence did not remain when measured after a longer period). In six trials at least one outcome showed such differences in favour of the control group. In 26 trials there was at least one outcome for which there was no difference between the contract and control groups.
There is not enough reliable evidence available to recommend the routine use of contracts in health services to improve patients' adherence to healthcare activities or other outcomes.
Background
For many treatments and health promotion strategies, participants need to take advantage of the advice, treatments and other actions offered by healthcare practitioners. A number of good studies and systematic reviews have evaluated interventions to improve patients' adherence to treatments (Haynes 2008; Rueda 2006). Haynes, for example, reports that interventions to improve short‐term adherence to medications are relatively successful, but interventions for chronic conditions tend to be complex and not very effective. One widely‐used approach is a contract between healthcare practitioners and participants. We examine here the use of contracts to improve adherence looking at the specific features of contracts.
Definition and characteristics
Contracts are defined as a mutual agreement between two or more parties that something shall be done by one or both (OED 2003). As a behavioural strategy aiming at improving patients' adherence, contracts refer "to a process of specifying a set of rules regarding some behaviour of interest and formalising a commitment to adhere to them" (Dunbar 1979). They are referred to as contracts, behavioural contracts or contingency contracts. Contracts have been used in a wide range of circumstances such as smoking cessation, breast self examination, hypertension, diabetes, rheumatic diseases, tuberculosis, hepatitis, for renal patients, and for people with psychiatric conditions.
In the social science literature, there is no consistent definition of contracts. This section aims to scope the features and concepts underpinning the use of contracts in health and draws from a wide range of research.
The following summarises the features of contracts when used as a strategy to increase adherence:
Formalisation. Contracts formalise the agreement of patients and/or healthcare practitioners to follow treatment, prevention or health promotion activities. These usually involve therapeutic activities (particularly adherence to prescribed drugs) but they also include: observance of appointments (Hayes 2000); lifestyle behaviours, such as smoking cessation (Resnikow 1997) and nutrition habits (Boehm 1997); and diagnostic actions, like breast self examination (Lierman 1994). Contracts are often written, but some examples of verbal contracts exist (Anderson 1982; Arnet 2000).
Parties to the contract. Contracts are most often established between patients and their physicians. There are examples of other parties being involved, such as nurses and patients (Boehm 1997), patients and selected partners from the household or the community (Keane 1984; Lierman 1994; Morisky 2001; Ossip‐Klein 1984), and even contracts with the patients themselves (a self‐commitment made explicit) (Brus 1998). We found one study of a tripartite contract: between the patient, the healthcare practitioner of a pain clinic and the primary care physician (Fishman 2002a).
Usually adults. In the literature, contracts primarily involve adult patients, although adolescents (Morisky 2001; Wysocki 1989) and children (Greenan‐Fowler 1987; Sherman 1991) have also been involved. The role of children is particularly delicate, since their decision capacity is limited and sometimes delegated to their carers, and their right to have access to information entails specific requirements to ensure their comprehension (Sanz 2003).
Contingency contracts
When contracts include a reward conditioned by the accomplishment of the contract clauses, they are referred to as contingency contracts: "a specifically negotiated agreement that provides for the delivery of positive consequences contingent on desirable behaviour" (Janz 1984). There are two main types of rewards (Christiensen‐S. 1985). 'Token economies', which were initially used as a behavioural therapy, are rewards from the healthcare practitioner in the form of tokens that can be exchanged for something of value (Hayes 2000; Wysocki 1989). Rewards may also involve the refund of a deposit ('deposit contract') (Chowdhury 1997; Molteni 1983; Paxton 1983). One study reported a self‐reward, where the patient states what s/he will do to reward him/herself (Neale 1991). Another study involved insurance refund policies based on measures of treatment success (Harzer 2000). Neither contingencies nor penalties seem to take place, however, if healthcare practitioners do not respect their terms in the contracts.
Ethical issues arise when access to treatment may be dependent upon patients' behaviour as specified in a contract (Biller 1999). Contracts have been used not only as behavioural therapy, but also to support decisions on the appropriateness of a given treatment. For example, one study described how compliance with a behavioural contract was used as a criterion to identify individuals with the potential to maintain a transplanted organ capably (Cupples 2001). The circumstances in which a patient can make a rational and autonomous choice, in the context of contracting, is also worthy of ethical consideration (Biller 1999).
For this review, contracts are defined as any type of agreement, verbal or written, by which one or both parties agree to a set of behaviours related to the care of a patient. Contracts may be established between healthcare practitioners and patients, between practitioners and carers, between carers and patients, or by a patient with him/herself. Contracts are intended to improve adherence to treatment, prevention and health promotion activities.
Theoretical models
Concordance and the relationship model
Compliance or adherence has been defined as "the extent to which a person's behaviour (in terms of taking medications, following diets or executing lifestyle changes) coincides with medical or health advice" (Haynes 1979a). The increasing use of the term 'adherence' instead of 'compliance' is due to the latter's negative and authoritarian connotations. Adherence implies the patient's active choice in following medical recommendations rather than passive co‐operation of obedience to them (Evangelista 2000). However, adherence is still rooted in a medical model, in which patients are expected to do what healthcare practitioners tell them. In this review, we use the term adherence in its most restricted sense, to designate the extent to which something that has been implicitly or explicitly agreed between healthcare practitioners and patients (for example, a treatment), actually happens, regardless of the type of relationship between patients and practitioners.
The term 'concordance' aims to reflect that patients/persons have self‐determination and control over what happens to them. Concordance means shared decision making and arriving at an agreement that respects patients' wishes and beliefs (Jones 2003). It has been argued that healthcare practitioners may also find that patients' difficulties in adhering to treatments ‐ such as those experienced by chronically‐ill patients with their treatments (for example, taking treatments consistently whilst suffering side effects) ‐ may be minimised in the context of a concordant relationship (Townsend 2003).
Some contracts depend on a relationship model. Contrary to the assumptions in a concordant relationship, the healthcare practitioner perspective predominates in the literature on behavioural contracts scrutinised so far. References to healthcare practitioners' obligations (like providing information or evidence‐based treatments) are generally missing. Contracts often appear not to be based on a relationship marked by shared decision making, but instead they place the responsibility of failing the terms of the contract on the patients' side. The literature around concordance is particularly relevant since it provides a critical perspective to understand the patient ‐ provider relationship, whatever form it takes (including contracts). With concordance, an essential component in a shared decision‐making model is that of mutual agreement (implicit or explicit) with the treatment decision (Charles 1997). This kind of agreement may indeed reinforce the mutual contribution of healthcare practitioners and patients to a successful treatment (Maher 2003). Furthermore, it has been argued that unless patients and doctors are collectively or jointly involved in the decision‐making process, sharing information and building up consensus, there is no basis for reaching an agreement on which a treatment can be implemented (Stevenson 2000). In a concordant consultation the patient and the healthcare professional participate as partners to reach that agreement (Cox 2004).
Impact on health
Low adherence may seriously compromise the effectiveness of therapeutic regimens. It has been reported that adherence may be as low as 10% in keeping appointments (number of appointments kept in relation to the total number of appointments scheduled), or may be between 40% and 60% in the case of adherence to long‐term medications (percentage of patients with presence of medications in body fluids or self‐assessed reporting of drug intake) (Sackett 1979). Poor adherence to treatment regimens has been associated with a reduction in treatment effectiveness, leading to worse health outcomes and even death (Cleemput 2002; Gordis 1979; Simpson 2006). The World Health Organization (WHO) report on adherence documents worse outcomes associated with poor adherence for conditions like hypertension, type‐2 diabetes and depression (WHO 2003). There is some evidence that the costs involved in treating non‐adherent patients are greater than those involved in treating adherent ones (Cleemput 2002; Heinssen 1995). In the United Kingdom (UK), it has been estimated that missed appointments resulted in an economic loss of 250 million pounds sterling per year (DPP 2003). However, adherence to potentially harmful treatments may also lead to adverse outcomes (Simpson 2006).
Advantages of contracts
What are the potential advantages of contracts over other interventions that seek to improve adherence and concordance? First, they could allow for better replication if they are standardised and do not include extensive training or educational components. Contracts may be cheaper to implement than other combined or more complex interventions, or even than supervised self‐administration of drugs (Keane 1984). Apart from that, in a case study, contracts have shown cost savings related to an increase in adherence and the rationalisation of the care provided (Heinssen 1995). For patients/participants, provided that the interventions used are effective, the benefits include health gains, psychological comfort (Jones 2003) and a better understanding of what they are expected to do and why.
Evidence base for improving adherence
Haynes reviewed the factors associated with the level of adherence to therapeutic regimens (Haynes 1979b). The type of disease seems to play a secondary role, except in specific conditions: adherence tends to be lower in some psychiatric disorders such as depression, for example. System or organisational issues such as referral delays, waiting times and appointment schedules have a stronger influence than the type of disease on the level of patients' adherence. In relation to the features of the therapeutic regimens, low adherence has been found almost constantly in treatments of longer duration and involving several drugs. Socio‐economic barriers, side effects of treatments and denial of the illness have also been related to poor adherence (Mellins 1992). Finally, the interaction between patients and healthcare practitioners is decisively important in ensuring that what has been explicitly or implicitly agreed, actually takes place. Effective communication of usage instructions for drugs, and the clinician's understanding of patients' concerns about their problems or treatment preferences, have been associated with an increase in patients' adherence and willingness to participate (Hulka 1979).
Interventions to increase adherence may address organisational issues, the simplification of therapeutic regimens, the interface between the patient and the healthcare practitioner, and patients' behaviour. Strategies to increase adherence to regimens have been systematically reviewed in general (Haynes 2008), and in relation to specific diseases, like tuberculosis (Volmink 2000; Volmink 2006), HIV/AIDS (Rueda 2006) or mental illness (Reda 2001), reporting the effects of these interventions on patients' adherence and on other outcomes. These interventions tend to be complex. Firstly, many different actors and activities may be involved. Educational interventions, for example, may involve physicians, other therapists, facilitators, educational materials, and different schedules and structures of the sessions. Secondly, some interventions are a combination of different strategies, such as patient instructions combined with visits to a specialist, or patient brochures together with group sessions. This complexity makes it very difficult to know which are the key elements that may have an impact on patients' adherence or on the improvement of health outcomes. Results from these reviews indicate that some strategies or combination of strategies may improve adherence or health outcomes, but their effects are not very remarkable overall when compared with the effort they require (Haynes 2008).
While this review focuses on a single strategy in the context of any health condition, several systematic reviews have assessed interventions to improve adherence or compliance in relation to specific conditions. Five included contracts. One was restricted to adherence to appointment keeping, and considered only randomised controlled trials written in English (Macharia 1992). Another assessed controlled studies, published in English language journals, of patients' adherence to therapeutic regimes (Roter 1998). Three other reviews were published in The Cochrane Library. One of them focused on tuberculosis (Volmink 2006), another on reminder packaging (Heneghan 2006) and yet another considered adherence to prescribed (self‐administered) medications only (Haynes 2008). No systematic review has addressed contracts as a strategy to improve patients' adherence to any kind of treatment, prevention or health promotion activity, regardless of the setting and the condition or disease affecting the patients.
Objectives
To assess the effects of contracts between patients and healthcare practitioners on patients' adherence to treatment, prevention and health promotion activities, the stated health or behaviour aims in the contract, patient satisfaction or other relevant outcomes, including health practitioner behaviour and views, health status, reported harms, costs, or denial of treatment as a result of the contract.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
After the initial publication of the protocol for this review, we amended the selection criterion for studies (which formerly included some study designs other than RCTs). Preliminary searching indicated that the number of randomised controlled trials potentially eligible for inclusion in this review was much larger than previously anticipated, thereby removing the need to examine studies providing less robust evidence. The 'Criteria for considering studies for this review / Types of studies' section was amended to include only RCTs (excluding quasi‐randomised trials, controlled before‐and‐after studies and interrupted time series analyses).
Types of participants
Patients or their carers, of any gender and age, with any health condition and in any health setting. The term 'patient' is used broadly to refer to any person undergoing diagnostic tests, or treatment, or participating in any illness prevention or health promotion initiatives.
Practitioners, including clinicians, nurses and any worker or service providing screening, diagnosis, therapeutics, rehabilitation, prevention or health promotion activities.
Types of interventions
Contracts concerning treatment, prevention and health promotion activities aimed at improving patients' adherence. Contracts included any verbal or written statement specifying at least one treatment, prevention or health promotion activity to be observed, and a commitment of adherence to it.
Contracts could take place between healthcare practitioners or services and patients or their carers, between patients and their carers, or between patients themselves (self‐commitment). Contracts could relate to any diagnostic procedure, therapeutic regimen, rehabilitation measure, general health advice, referral instruction, or any other activity or combination of activities involved in the management of patients.
Explicit rewards (like tokens, cash or social benefits) may or may not have been present. Self‐management was included, providing that self‐management appears to be supported by any form of contracting.
The control was any intervention (such as instructions, education, incentives or reminders) or combination of interventions, aimed at improving patients' adherence; or no intervention. We excluded studies comparing different modalities of contracts.
We included studies of multifaceted interventions provided that a given modality of contract was present in the intervention but not in the control group.
Types of outcome measures
Primary outcomes
Patients' adherence or change in behaviour related to adherence (e.g. patients' adherence to treatment regime, to undergo a diagnostic procedure, to participate in a health promotion programme, consistency with agreed targets, attendance, participation number and rates, length or duration of participation, healthcare practitioners' adherence to agreed specifications).
Secondary outcomes
Patients' participation in the contractual process (such as inclusion of patients' values and preferences) and degree of shared decision making where alternative treatment options are present, assessed through qualitative statements or scales.
Outcomes of agreed aims stated in the contracts, both for patients and for healthcare practitioners.
Patients' satisfaction with the contracting process, assessed either qualitatively or through scales. This includes satisfaction with the level of knowledge about the healthcare process, reduction in the level of distress and other psychological outcomes reported.
Healthcare practitioners' observance of contract terms and appraisal of the contracting process.
Health status measures: all outcomes consistent with, or relevant to, the aims/specifications of contracts (e.g. for treatment, prevention or health promotion, including mortality and morbidity outcomes, improvement in the control of chronic conditions and relief of symptoms).
Harms associated with adhering to proposed treatment or health promotion activity, (e.g. reported side effects, defaulted treatment, and difficulties associated with maintaining treatment or health promotion activities).
Costs or savings incurred by patients, healthcare practitioners, services or other institutions (e.g. insurance companies) derived from adherence or non‐adherence to healthcare activities.
Denial or deferral of treatment.
A post‐hoc outcome related to the utilisation of health services has been added, as it has been found in one of the trials and we think it is relevant in this review.
Although an association between adherence to drug therapy and positive health outcomes has been shown (Simpson 2006), this does not necessarily mean that good adherence to medication will always predictably lead to better health outcomes (Haynes 2008). However, we still think that it is of value to include studies with only adherence‐related outcomes, because certainly good adherence may be a pre‐requisite, although not the only one, for achieving good health outcomes.
Search methods for identification of studies
We sought studies in any language regardless of their publication status (published, unpublished, in press and in progress).
We searched the following electronic databases using specific search terms in combination with the search strategy for identifying trials, as detailed in Appendix 5b of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006):
Cochrane Consumers and Communication Review Group's Specialised Register (in May 2004).
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2004, issue 1) .
MEDLINE (1966 to May 2004).
EMBASE (1980 to May 2004).
PsycINFO (1966 to May 2004).
CINAHL (1982 to May 2004).
Dissertation Abstracts. A: Humanities and Social Sciences (1966 to May 2004).
Sociological Abstracts (1963 to May 2004).
UK National Research Register (2000 to May 2004).
C2‐SPECTR, Campbell Collaboration (1950 to May 2004).
We present the full search strategy for MEDLINE (Ovid) at Appendix 1. We searched the reference lists of relevant studies identified by the search.
Data collection and analysis
Study selection
One author (XBC) assessed the titles and abstracts of potentially‐relevant studies against the review inclusion criteria. If a study could not be excluded on the basis of the title or abstract alone, we obtained full papers. Two authors (XBC and KA) assessed potentially‐relevant papers for inclusion independently against the review inclusion criteria. We resolved disagreements through discussion and, if an agreement was not reached, referred to a third author (PG). Reports were scrutinised for multiple publication. We excluded potentially‐relevant studies that did not meet the inclusion criteria, giving the reasons for exclusion in the table Characteristics of excluded studies. We attempted to contact some study authors for clarification where information was missing, but the age of some of the trials, together with authors' resource constraints, meant that this was not always possible. We aim to increase author contact for future updates of this review.
Assessment of methodological quality
Two authors (XBC and KA) assessed independently the quality of studies (see criteria below). This process was not blind in relation to the trial authors, their institutions and journals.
We used a form to guide the assessment of methodological quality, and classified each quality component as 'adequate', 'inadequate' or 'unclear'. Disagreement was resolved by discussion with the third author (PG).
The criteria applied to assess the methodological quality were as follows:
Method of randomisation: rated 'adequate' if the method used was described and the resulting sequences were unpredictable (e.g. random numbers, drawing of lots or envelopes, tossing a coin); rated 'inadequate' if the sequences could be related to non‐random factors (e.g. record number, date of birth); rated 'unclear' if the description did not allow us to judge the method of randomisation.
Concealment of allocation: rated 'adequate' if participants and investigators could not foresee the assignment (e.g. central randomisation remote from trial location; sequentially numbered, opaque, sealed envelopes); rated 'inadequate' if participants and investigators enrolling participants could foresee the upcoming assignment (e.g. open allocation schedule; unsealed or non‐opaque envelopes); rated 'unclear' if the description did not allow us to judge allocation concealment. In the table Characteristics of included studies allocation concealment was reported as: adequate (A), unclear (B), inadequate (C), or that allocation concealment was not used (D) as a criterion to assess validity (Higgins 2006, chapter 6.3).
Blinding of practitioners: rated 'adequate' if it was reported that practitioners or researchers (those offering the intervention) were blind to who was in each group; rated 'inadequate' if practitioners or researchers knew the participants' group, and this was stated or could be clearly inferred from the text; rated 'unclear' if the description did not allow us to judge blinding of practitioners.
Blinding of participants: rated 'adequate' if participants did not know to which group they belonged; rated 'inadequate' if participants knew to which group they belonged; rated 'unclear' if the description did not allow us to judge blinding of participants.
Blinding in the assessment of outcomes: rated 'adequate' if trial authors explicitly stated that the primary outcome variables were assessed blindly; rated 'inadequate' if outcome(s) were not assessed blindly; rated 'unclear' if the description did not allow us to judge blinding of outcome assessment.
Baseline measurements: rated 'adequate' if baseline measurements were reported and there were no significant differences between groups; rated 'inadequate' if baseline measurements were reported and there were significant differences between groups; rated 'unclear' if baseline measurements were not reported.
Loss to follow up: rated 'adequate' if outcome measures were explicitly obtained for 80% or more of professionals, subjects, patients or episodes entering the study; rated 'inadequate' if outcome measures were obtained for less than 80% of professionals, subjects, patients or episodes entering the study; rated 'unclear' if it was not reported or it was impossible to estimate.
Consumer participation: rated 'adequate' if there was any mention of the involvement of consumers in the design, implementation or interpretation of the research; rated 'inadequate' if it was explicitly stated that consumers did not participate in any stage; rated 'unclear' if nothing was reported.
The assessment of methodological quality for each included study is reported in Table 7.
Table 1.
Assessment of methodological quality
| Study | Randomisation method | Allocation concealment | Baseline measures | Practitioners blind | Participants blind | Outcomes blind | Follow up | Consumers involved |
| Aragona 1975 | Unclear | Unclear | Adequate | Unclear | Unclear | Unclear | Adequate | Unclear |
| Barrera 1977 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Binstock 1988 | Unclear | Unclear | Adequate | Unclear | Unclear | Unclear | Adequate | Unclear |
| Brockway 1977 | Unclear | Unclear | Inadequate | Unclear | Unclear | Unclear | Adequate | Unclear |
| Burkhart 2002 | Adequate | Unclear | Inadequate | Unclear | Unclear | Unclear | Adequate | Unclear |
| Calsyn 1994 | Unclear | Unclear | Inadequate | Unclear | Unclear | Unclear | Adequate | Unclear |
| Claydon 1997 | Unclear | Unclear | Adequate | Inadequate | Adequate | Adequate | Adequate | Unclear |
| Curry 1988 | Adequate | Unclear | Unclear | Unclear | Unclear | Unclear | Adequate | Unclear |
| Flanders 1985 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Craighead 1989 | Unclear | Unclear | Adequate | Unclear | Unclear | Unclear | Inadequate | Unclear |
| Haber 1993 | Unclear | Unclear | Adequate | Inadequate | Adequate | Inadequate | Adequate | Unclear |
| Hammond 1999 | Unclear | Unclear | Inadequate | Inadequate | Adequate | Adequate | Inadequate | Unclear |
| Hoelscher 1986 | Unclear | Unclear | Adequate | Unclear | Unclear | Adequate | Adequate | Unclear |
| Keane 1984 |
Data Extraction
Trials were distributed among two authors (XBC and KA) for data extraction. The statistics editor and statistics assistant of the Cochrane Consumers and Communication Group checked the data extraction. Data extracted included the study design, methods, participants, interventions, co‐interventions and outcomes. Data extracted to describe the modality of contracts included: formalisation and duration of contracts, parties (categorised as practitioner, participant/patient, carer (including peers and significant others) and other), treatment, prevention and health promotion activities involved, and contingencies. We also extracted data on the profile of trial participants.
We extracted the following data on outcomes (for all parties, such as for children and parents): measures of adherence to therapeutic regimens and use of services; adherence of healthcare practitioners to the terms of the contracts; penalties and rewards; quantitative measures or qualitative data describing the level of shared decision making; measures of satisfaction with the process; expectations and psychological distress; healthcare practitioners' understanding and behaviour in relation to contracts; health status data, such as improvement in clinical parameters or prognosis; cost information, detailing (where possible) the way costs have been estimated; and data on harms derived from the adherence or lack of adherence to treatment/s.
Data Analysis
Where no intention‐to‐treat (ITT) analysis had been carried out, we have tried to extract data to do it. Percentage loss to follow up has been presented as reported, or calculated if the number of selected individuals did not match the number of individuals whose data has been analysed. For binary outcomes we recorded the number of participants experiencing the event in each group and calculated the odds ratios. For continuous outcomes we extracted the arithmetic means and standard deviations (SD).
The main features of included studies have been presented in the table Characteristics of included studies, which also includes the country, setting, health area or problem, recruitment mechanism, sample size of participants randomised and main features of contracts. Additional tables Table 8; Table 9; Table 10; Table 11 (one for each main group of health problems or areas) describe the number of participants included in the analysis (which may differ to the number of participants randomised), the interventions, controls and outcomes. For each study, outcomes have been placed in three columns depending on whether there were statistically significant differences favouring the intervention group, the control group or there were no differences, respectively.
Table 2.
ADDICTIONS: Outcomes for each individual study and statistical significance
| Study | Number analysed | Contract details | Co‐intervention | Control description | Control details | Outcomes: favouring intervention | Outcomes: favouring control | Outcomes: no difference |
| Brockway 1977 | 27 | Eliminate smoking in two situations per week. Subjects monitored their smoking behaviour in detail / multi‐session smoking cessation programme. Teaching of relaxation. Information on the effects of stopping smoking. | Yes | Routine | No smoking cessation programme. | Mean number of cigarettes smoked at end of treatment, 3 and 6 months follow up. | Mean number of cigarettes smoked at 12 months follow up. | |
| Calsyn 1994 | 353 | Contracts written depending on achievement of abstinence goals. ‐ Group (1) Medication only: saw counsellor to complete standard treatment. ‐ Group (2) Standard: counselling sessions and optional drug education classes. ‐ Group (3) Enhanced: as per group (2) plus relapse prevention skill training group and weekly group treatment. | Yes | Complex | Three groups (4), (5) and (6), replicating the intervention conditions but without contingency contracting. | ‐ Time with positive urine analyses for opiates (groups 1 versus 4). ‐ Positive urine analyses after 9 week stabilisation period for opiates. ‐ Positive urine analyses after 18 months, (a) regardless of the substance, (b) for opiates and (c) for cocaine. ‐ Time out of treatment before readmission. | ‐ Lower discharge rate in control group. | ‐ Time with positive urine analyses for cocaine. ‐ Retention in treatment (significance not reported). ‐ Positive urine analyses after 9 weeks stabilisation period for cocaine. |
| Curry 1988 | 139 | Absolute abstinence / contingency contracting. | Yes | Complex | Relapse prevention: cold turkey withdrawal, identifying high risk situations, etc. | Percentage of participants abstinent (both for all participants randomised, and for only those who began the treatment) at several periods (post‐treatment up to 1 year). | ||
| Keane 1984 | 25 | ‐ Group (1) Contract / recording. ‐ Group (2) Contract / recording + instructions for positive reinforcement. | Yes | Complex | ‐ Group (3) Explanations in relation to disulfiram (Antabuse); phone calls to check on use of disulfiram (Antabuse) and aid in resolving difficulties. | ‐ Three months of disulfiram (Antabuse) dispensed by the pharmacy. ‐ disulfiram (Antabuse) intake reported by other (significance not reported). ‐ Aftercare sessions attended (significance not reported). | ||
| Lash 1998 | 40 | Aftercare orientation session plus aftercare participation contract. | Yes | Routine | Videotape of motivational speaker on aftercare. | ‐ Mean number of sessions attended. ‐ Number of subjects attending at least one aftercare group session | ||
| O'Farrell 1984 | 36 | ‐ Group (1) Husband takes disulfiram (Antabuse). Wife observes and records it. In return she will not mention any past drinking or any fears about future drinking. | Yes | Complex | ‐ Group (2) Interactional group: catharsis, ventilation, sharing of feelings. ‐ Group (3) no treatment. | Satisfaction outcomes, ability to solve problems, adherence to sessions (significance level not reported). (Data reported for group 1 and group 2 only). | ||
| Ossip‐Klein 1984 | 50 | Posting the prompt calendar; attending aftercare; calling the Alcohol Program if unable to attend. | No | Routine | Only telephone prompt. | Percentage attendance aftercare sessions 1, 3, 4 and 6 (6 months). | Percentage attendance aftercare session 2, 5, 7 and 8. | |
| Piotrowski 1999 | 102 | Contingency contracting for absence of illicit drugs. | No | Routine | Random tests and feedback only. | ‐ Longest period with continuous abstinence at 90 to180 days. ‐ Longest period with abstinence for benzodiazepines and marijuana. | ‐ Substance free samples (proportion of subjects). ‐ Longest period with continuous abstinence at 30 to 60 days. ‐ Longest period with abstinence for all substances but benzodiazepines and marijuana. ‐ Total costs of treatment at 1 to 4 months. | |
| Poole 1981 | 75 | Group (1) Rapid smoking / relaxation / contracting. | Yes | Behavioural | ‐ Group (2) Rapid smoking session. ‐ Group (3) Rapid smoking / relaxation. ‐ Group (4) Contingent Rapid smoking. | ‐ Time remaining abstinent (measured by self‐reported daily cigarette consumption) similar between groups (measured at any time period from 1 week to 12 months). ‐ Cigarette consumption compared with baseline smoking, from 1 week to 12 months follow up. | ||
| Vinson 2000 | 69 | Produced by the patient using a list of options in a computer programme. | No | Routine | Screening and baseline assessment. | Change in Alcohol Use Disorders Identification Test (AUDIT) scores at 12 months. (Note: Addiction Severity Index (ASI) scores not reported for intervention and control group separately). |
Table 3.
HYPERTENSION: Outcomes for each individual study and statistical significance
| Study | Number analysed | Contracts details | Co‐interventions | Control description | Control details | Outcomes: favouring intervention | Outcomes: favouring control | Outcomes: no difference |
| Binstock 1988 | 112 | ‐ Group (1) Contracts + educational program. ‐ Group (2) Contracts + educational programme + BP measurement at home + calendar pills. | Yes | Educational | ‐ Group (3) Bi‐monthly educational program. ‐ Group (4) Educational + BP measurement at home. ‐ Group (5) Calendar pills. | Change of blood pressure from baseline to 1 year follow up (not significant differences between groups 1, 2, 4 and 5). | ||
| Hoelscher 1986 | 50 | ‐ Group (1) Contracts / group relaxation. | Yes | Complex | ‐ Group (2) Individual relaxation. ‐ Group (3) Group relaxation. ‐ Group (4) Waiting list. | Cost‐effectiveness (1 versus 2). | Compliance with relaxation practices (1 versus 3). | Blood pressure reduction at 6 and 10 weeks (not significant 1 against 3) |
| Schulman 1980 | 91 | ‐ Group (1) Contract with behavioural goals. | Yes | Educational | ‐ Group (2) Routine / education booklets. ‐ Group (3) Routine. | Active Patient Orientation scores (see text for further explanations). Availability of treatment resources score (1 versus 2 and 3). Facts related to the management of hypertension (1 versus 3). (Patients' perceptions of the treatment rationales or facts the staff shared with them, and of the resources available, respectively.) | Facts index (1 versus 2). | |
| Swain 1981 | 115 | As above | Yes | Educational | As above | Change in knowledge score (1 versus 2). Subjects discontinuing treatment. Diastolic blood pressure controlled. |
Table 4.
OVERWEIGHT: Outcomes for each individual study and statistical significance
| Study | Number analysed | Contracts details | Co‐intervention | Control description | Control details | Outcomes: favouring intervention | Outcomes: favouring control | Outcomes: no difference |
| Aragona 1975 | 12 | ‐ Group (1) Contracts plus exercise programme, nutritional information, food diary. ‐ Group (2) like Group (1) plus reinforcement (deposit). | Yes | ‐ Group (3) Routine | Weight change from start to end treatment and at 8 weeks follow up. | |||
| Craighead 1989 | 62 | Group (1) Contracted exercise / written lessons. | Yes | Complex | Instructions plus ‐ Group (2) Supervised exercise. ‐ Group (3) Minimal contact. | ‐ Among completers of the 12 week treatment, weight loss measured at 12 weeks (groups 1 versus 3). ‐ Among completers of follow up (1 year), weight loss measured at 12 weeks (1 versus 3) | ‐ Among completers of the 12 week treatment, weight loss measured at 12 weeks (groups 1 versus 2). ‐ Among completers of follow up (1 year), weight loss measured at 12 weeks (1 versus 2) ‐ Among completers of follow up (1 year), weight loss measured at 12 months (1 versus 2) Harvard step test fitness score pre‐post group 2 (not significant in the others). | ‐ Among completers of follow up (1 year), weight loss measured at 12 months (1 versus 3). ‐ Treatment self‐reported as helpful (group 1 versus 2). |
| Murphy 1982 | 97 | ‐ Group (1) Sessions attended alone: 1 party contract. ‐ Group (2) Alone: 2 Parties. ‐ Group (3) Couple: 1 Party. ‐ Group (4) Couple: 2 Parties. | Yes | Complex | ‐ Group (5) Support group. ‐ Group (6) Waiting list. | Mean weight loss, percentage excess weight loss, weight reduction index all at 10 weeks comparing groups 1 to |
Table 5.
MISCELLANEOUS: Outcomes for each individual study and statistical significance
| Study | Number analysed | Contracts details | Co‐intervention | Control description | Control details | Outcomes: favouring intervention | Outcomes: favouring control | Outcomes: no difference |
| Barrera 1977 | 24 | Snake phobia. ‐ Group (1) Contract and self‐administered desensitisation. | Yes | Routine | ‐ Group (2) Self‐administered systematic desensitisation (SSD). ‐ Group (3) Placebo bibliographic programme. | Sessions attended and time spent studying the materials (group 2 versus 1). | Post‐test or follow‐up score of any outcome. | |
| Burkhart 2002 | 42 | Asthma. Contract for Peak Expiratory Flow Rate (PEFR) monitoring, reinforcement, tailoring, reminders. | Yes | Routine | Training in using peak flow meter. | Adherence to PEFR monitoring; asthma episodes. | ||
| Claydon 1997 | 75 | Contact lenses. Teaching checklist, complications poster, care regimen video, regimen poster, booklet, appointment reminder, telephone call. Contract. | Yes | Routine | Provision of contact lenses, solutions, basic instructions and aftercare. | All outcomes (e.g. washing hands or rinsing lenses). | ||
| Flanders 1985 | 42 | Acne. ‐ Group (1) Non‐contingent contract. ‐ Group (2) Contingent contract both with education + self monitoring medication card. | Yes | Complex | ‐ Group (3) Education and self‐monitoring card. ‐ Group (4) Waiting list. | Compliance. Number of acne lesions. | ||
| Haber 1993 | 64 | Healthy diet. Contracts, peer support group intervention and health education classes. | Yes | Educational | Health education classes. | Increase in fibre, salt limited. | Limiting fats, sweets; practice of stress management techniques and exercises. | |
| Hammond 1999 | 35 | Arthritis. Contracts, Joint Protection education group. | Yes | Routine | No intervention (later received active intervention) | Joint protection behaviour score (before cross‐over); self reported joint protection practice. | Joint protection behaviour score (after cross‐over). Joint protection knowledge. Health related outcomes. | |
| Litzelman 1993 | 395 | Diabetes. Contracts and educational sessions. | Yes | Routine | Two health outcomes (e.g. ulcers); five behaviour outcomes (e.g. wash feet); four items in physician documentation (e.g. ulcers recorded). | Five health outcomes (e.g. ingrowing nails); seven behaviour outcomes (e.g. trimmed nails) and six items in physician documentation (e.g. record of foot deformities). | ||
| Mayer 1991 | 36 | Breast self examination (BSE). Contracts, workshops (training on BSE), prompting / reminder options. | Yes | Educational | Workshops and mail prompts. | Breast self‐examination frequency; frequency of being prompted. | ||
| McLean 1973 | 20 | Depression. Contract related to husband and wife behaviour and training in social learning principles, course in immediate feedback. | Yes | Routine | Monitoring of the course of their depression, plus usual care. | Improvement in target behaviours at mid treatment, end treatment and 3 months follow up. Decrease of negative reaction at end treatment. | ||
| Morgan 1988 | 60 | Diabetes. Teaching / contracts. | Yes | Educational | Formal teaching plan on diabetes and diet. | Knowledge score change from week 1 to 8. | Weight loss, fasting blood glucose and glycosylated haemoglobin decrease in the 8 week period. | |
| Morisky 2001 | 794 | Tuberculosis. ‐ Group (1) Only contracts. ‐ Group (2) Counselling / contracts. | Yes | Complex | ‐ Group (3) Counselling. ‐ Group (4) Routine. | Completion of treatment comparing (1) and (2), favouring (2). | Completion of treatment comparing (1) and (3), and (1) and (4). | |
| Putnam 1994 | 60 | Acute infection. Self‐commitment. | No | Routine | Usual care. | Adherence based on pill count. | Self‐reported adherence; additional prescriptions received. | |
| Wurtele 1980 | 1946 | Tuberculosis. ‐ Group (1) Written and verbal commitment to return for the skin test. | No | Routine | ‐ Group (2) Verbal commitment. ‐ Group (3) No commitment. | Compliance: group 1 better than 2, and 2 better than 3. |
Trials were all too diverse in terms of co‐interventions, control groups, features of contracts, outcomes and settings to try any grouping by those criteria. Although the initial sub‐group analyses options included health status outcomes, presence and type of contingencies, degree of shared decision making and type of healthcare activity, we decided that the clearest way to group trials was by health area, because slightly more than half of the included trials could be grouped into three health areas (addictions, hypertension, and weight control). The remaining trials examined a range of conditions and are listed as 'miscellaneous' in our grouping. Data were presented by means of graphics only where data were complete (numbers in all groups available for categorical variables, and numbers in groups, means and standard deviations for continuous variables).
Consumer participation
Given that this review was not limited to any particular condition, we sought input from consumers or patients whose health experiences were not restricted to a single disease group and with experience or involvement in issues related to the relationship between patients and healthcare practitioners. Consumer participation was ensured in the protocol stage, and in the development of the review, and will be taken into account in future updates.
The protocol for this review, together with a user‐friendly questionnaire in electronic format to guide the process, was sent to a number of consumers for comments. Feedback was received from the following people and institutions: a social sciences and gender specialist working as a Community Research and Training Consultant, who is familiar with consumers' points of view (the Gender and Health Group, Liverpool School of Tropical Medicine, Liverpool, UK), and the Director of Developing Patient Partnerships (London, UK). The Cochrane Consumers and Communication Review Group involved two other consumers as external peer‐reviewers of the protocol, and one consumer as an external peer‐reviewer of the review. Additionally, several consumers involved in The Cochrane Collaboration provided feedback directly to the review authors at both protocol and review stages. Suggestions from consumers have been incorporated into the protocol and review as much as possible.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
As a type of intervention designed to help shape the relationship between patients, carers and practitioners, contracts are extremely complex, poorly defined and described, and evaluated in many different formats and ways. The lack of a consistent definition and common features, and the variation in trials undertaken, meant that we had to select one sensible way to present the results. The table Characteristics of included studies offers a summary of the following features of the included studies:
study design;
participants, including: country, setting, health problem or area, method of recruitment, type of participants and number of participants being randomised;
Intervention, including characteristics of contract (form, parties, type of incentives and existence of co‐interventions) and groups to which participants were allocated.
outcomes.
We outline below the main elements of the studies included in this review, in terms of the selection of studies; location and setting; health problems addressed; participants; interventions and control groups.
Results of the search
The search strategy retrieved a total of 4191 titles and abstracts. Of those, 768 items were duplicates, 3348 were irrelevant, and 75 appeared to be relevant. Of those 75 that were potentially relevant, we excluded 43 papers and included 32.
Included studies
Two pairs of trials referred to the same trials presenting data from two different follow‐up periods: Piotrowski 1999 and Hartz 1999 being one pair, and Calsyn 1994 and Saxon 1996 the other. The results of these studies are reported under the study identifiers Piotrowski 1999 and Calsyn 1994, respectively. Schulman 1980 seemed to be based in the same setting as Swain 1981, although it was unclear whether the data analysed came from the same set of patients. For the moment, we have reported the results as two trials but aim to clarify this in the future. The final number of included trials is 30. The dates of published trials ranged from 1973 to 2001.
All included studies were randomised controlled trials. Six of them (20%) used modified randomisation techniques (stratified and cluster randomisation).
Location (country and setting)
The studies were based in the USA (26), UK (2), Canada (1) and Australia (1). The main settings of trials were:
Specialised services (7): clinics specialising in providing care for addictions, a geriatric centre and an optical centre;
Primary health care (5);
Hospital (2);
Other settings (9) including specially set up programmes for substance abuse, a weight loss programme for young girls and other community based trials.
In seven (7) trials the setting could not be identified.
Health problems or areas
The included trials covered a wide range of health problems or areas, including;
Addictions (10): these included alcohol (5 trials), smoking (3 trials) and opiates (2 trials);
Hypertension (4);
Weight control (3);
Miscellaneous (13) included: diabetes, tuberculosis, breast self examination, healthy diet for the elderly, acne, depression, fear desensitising, acute antibiotics treatment, eye care, rheumatoid arthritis, and asthma.
Participants
Participants in all trials were people receiving care for a disease or who were targets for preventive interventions. In 13 trials they were recruited from the health system (patients receiving care, attending ambulatory services or referred). Eleven trials recruited participants using adverts, two trials used both methods, and another trial recruited college students. The recruitment method was not described in one trial.
The median number of participants per group was 21 (interquartile range 24 subjects). All trial participants were adults except in: Aragona 1975 (overweight children); Burkhart 2002 (children with asthma); Wurtele 1980 (screening for tuberculosis) where the age of participants ranged from 5 to 76 years; and Morisky 2001 (adolescents treated for latent tuberculosis, aged 11 to 19).
Fourteen trials (47%) compared two groups, eight trials (27%) had three groups, five trials (17%) had four groups, one trial (3%) had five groups and two (7%) trials had six groups.
Intervention: characteristics of contracts
Format
Contracts were written in 25 trials (83%), and in the other 5 trials (17%) their format was not stated. Only four trial reports (13%) included a sample of the contract form (Litzelman 1993; Morgan 1988; O'Farrell 1984; Ossip‐Klein 1984).
Parties
Contracts were mainly established between two parties: between participants or patients and healthcare practitioners in seven trials (23%), between participants or patients and carers, peers or significant others in nine trials (30%), and between healthcare practitioners and carers in one trial (3%). In four trials (13%) contracts were tripartite between patients, carers and healthcare practitioners. Two trials (7%) examined a self‐contract. In the other seven trials (23%) the parties involved in the contracts were not reported. See the 'Characteristics of included studies table for details on each particular trial.
Terms and incentives
Terms
The terms of the contracts included:
Stopping or reducing substance abuse (alcohol, opiates, tobacco) (Calsyn 1994; Curry 1988; Piotrowski 1999; Poole 1981).
Posting a prompt calendar in a prominent location, plus attending after care sessions and calling the alcohol programme in advance if unable to attend (Ossip‐Klein 1984).
Recording disulfiram (Antabuse) intake which was mailed to the treatment programme monthly (Keane 1984).
Attending sessions (Brockway 1977; Lash 1998).
Keeping record of drinks and limiting alcohol intake (Vinson 2000).
Wives of participants observing and recording whether disulfiram (Antabuse) was taken by their husbands, and in return they avoid mentioning any fears of their husband's future drinking, with instructions on when to search for medical care (O'Farrell 1984).
Practicing muscular relaxation (Hoelscher 1986).
Exercising (Craighead 1989; Murphy 1982; Swain 1981).
Changing eating habits (Morgan 1988; Murphy 1982; Swain 1981).
Setting goals for children's weight loss (Aragona 1975).
Working on a manual for phobia desensitising (Barrera 1977).
Following written instructions for contact lens care, reasons for care and goals for successful care (Claydon 1997).
Monitoring Peak Expiratory Flow Rate (PEFR) (Burkhart 2002).
Returning for tuberculosis skin‐test reading (Wurtele 1980).
Reminding about breast self examination (BSE) (Mayer 1991).
Monitoring use of hands, and pain (Hammond 1999).
Taking medication (Flanders 1985, Morisky 2001, Putnam 1994).
Foot care behaviours (Litzelman 1993).
Following specified behaviours towards partners (McLean 1973).
One trial (3%) did not explicitly report the terms of the contract (Binstock 1988).
Some of the contract terms included adherence to treatment (e.g. return for tuberculosis skin‐test reading). These are considered as outcomes if they are presented as such in the studies, regardless of whether they are also part of the contract's terms.
Incentives
In 21 trials contracts had incentives attached to them, contingent to the fulfilment of the contract terms. Incentives were of several types:
Five trials (17%) featured deposits. Participants delivered a given amount of money to the researchers or healthcare practitioners, which was then totally or partially reimbursed upon completion of the terms of the contract (Aragona 1975; Brockway 1977; Craighead 1989; Mayer 1991; Poole 1981).
Three trials (10%) incorporated tokens or goods, such as cash credits to be exchanged for items that participants chose, or selection of a gift (Flanders 1985; Murphy 1982; Piotrowski 1999).
Other incentives were used in 13 trials (43%), as follows: changes in methadone dosage (Calsyn 1994); special meals and recreational activity (Ossip‐Klein 1984); rewarding activities (Barrera 1977; Hoelscher 1986); self‐defined rewards (Binstock 1988; Burkhart 2002; Morgan 1988), change of partner behaviour (McLean 1973), praising and stickers (Burkhart 2002), punishment of sending money to someone participants disliked (Curry 1988), random reward (Flanders 1985) and unspecified rewards (Hoelscher 1986; Putnam 1994; Swain 1981; Wurtele 1980).
Contracts in nine trials (30%) had no incentives attached to them.
Co‐interventions
Twenty‐five trials (83%) had co‐interventions (some of them had more than one). It was not always clear whether an intervention was part of the contract arrangement, or was actually a co‐intervention. For example, the terms of the contract in Ossip‐Klein 1984 included posting a prompt calendar to remember specific tasks, but this reminder mechanism could also be seen as a co‐intervention. Co‐interventions included:
Counseling/education/instructions (18 trials): Aragona 1975; Barrera 1977; Binstock 1988; Calsyn 1994; Claydon 1997; Curry 1988; Craighead 1989; Haber 1993; Keane 1984; Lash 1998; Litzelman 1993; McLean 1973; Morgan 1988; Morisky 2001; Murphy 1982; Schulman 1980; Swain 1981; Vinson 2000.
Training (skills or behaviours) (11 trials): Aragona 1975; Binstock 1988; Brockway 1977; Burkhart 2002; Calsyn 1994; Curry 1988; Hammond 1999; Hoelscher 1986; Mayer 1991; O'Farrell 1984; Poole 1981.
Reminders (4 trials): Burkhart 2002; Haber 1993; Mayer 1991; Morgan 1988.
Group support/treatment (2 trials): Calsyn 1994; Haber 1993.
Monitoring or recording of medication taken, problems related to taking medication (2 trials): Flanders 1985; Keane 1984.
Goal setting (1 trial): Calsyn 1994.
Control groups
Control groups consisted of routine care in 14 trials (47%). Non‐routine control groups included the following interventions:
Counseling/education/instructions (8 trials): Binstock 1988; Calsyn 1994; Craighead 1989; Haber 1993; Keane 1984; Morgan 1988; Morisky 2001; Swain 1981.
Group support / treatment (5 trials): Curry 1988; Hoelscher 1986; Mayer 1991; Murphy 1982; O'Farrell 1984.
Training (5 trials): Binstock 1988; Calsyn 1994; Curry 1988; Hoelscher 1986; Poole 1981.
Reminders (1 trial): Mayer 1991.
Others (2 trials): cognitive re‐structuring, role playing (Curry 1988); supervised exercise (Craighead 1989).
Risk of bias in included studies
Eight methodological quality criteria were applied to each trial (see 'Methods of the review / Assessment of methodological quality', for details). None of the trials met 5 or more of the 8 methodological quality criteria; 1 trial met 4 criteria, 3 trials met 3 criteria, 6 met 2 criteria, 11 trials met a single criterion and the remaining 9 trials met none of the criteria. The assessment of methodological quality for each included study is reported in Table 7.
Method of randomisation and concealment of allocation
The randomisation mechanism to allocate participants into groups was appropriately reported in three trials (Burkhart 2002; Curry 1988; Vinson 2000). In the other 27 trials it was not possible to determine the randomisation mechanism, although none gave any evidence of utilising a quasi‐experimental rather than truly randomised study design.
Only two trials mentioned a method which allowed for concealment of allocation (Ossip‐Klein 1984; Vinson 2000); in 28 trials (94%) allocation concealment was unclear.
Baseline measurements
Baseline measurements were reported in 24 trials. No differences in baseline measurements were reported in 16 trials, although only 9 of them showed baseline data. The other eight trials reported some differences (six of them showing data).
Blinding
This behavioural intervention is difficult to blind to practitioners and participants. Only four trials reported blinding of practitioners or researchers (Litzelman 1993; Ossip‐Klein 1984; Putnam 1994; Swain 1981). In 22 trials blinding was not reported and in the other 4 trials it was clearly stated that practitioners were not blind to group allocation.
In 3 trials participants were blind to the allocated intervention (Claydon 1997; Haber 1993; Hammond 1999), and the other 27 trials did not mention blinding of participants. In Claydon 1997, it should be noted, patients were unaware of being participants in a trial.
Blinded assessment of outcomes was reported in 6 trials (Claydon 1997; Hammond 1999; Hoelscher 1986; Litzelman 1993; Vinson 2000; Wurtele 1980). In 23 trials it was unclear, and 1 trial reported that outcome assessors were not blind to group allocation.
Follow up
Loss to follow up was less than 20% (rated as 'adequate') in 19 trials, more than 20% (rated as 'inadequate') in 4 trials, and could not be determined in the other 7 trials.
Community or user involvement
None of the trials reported any participation of community members or users in the design, implementation or interpretation of the research, beyond the involvement expected from a behavioural intervention.
Data on outcomes
Nine of the 30 trials provided enough data to estimate statistical differences between groups (Craighead 1989, Lash 1998, Litzelman 1993, McLean 1973, Morisky 2001, Ossip‐Klein 1984, Piotrowski 1999, Poole 1981, Putnam 1994). The presentation of numerical data was of poor quality: some statistical significances were just mentioned in the text without P values; others had P values but not the statistical parameter used (for example, F, t) or their values; some did not show the number of subjects included in the analyses of each group; and sometimes comparisons of more than one intervention group were pulled together against more than one control group pulled together as well.
Sample size
Sample sizes were generally small. The median sample size per group was 21 (interquartile range 24), and only two trials had more than 100 subjects in each group. With this very limited sample size it is difficult to have the power to estimate relatively small differences between groups.
Effects of interventions
The numerous outcomes were difficult to group in terms of their meaning, methods of assessment and times of the assessments. Therefore, it seemed impractical to attempt any pooling of data for meta‐analysis. However, for those outcomes where data were complete (for example, standard deviations included when estimating means, or the number of subjects included in the analyses of each group), and where appropriate, we entered data into RevMan Analyses and produced forest plots, as noted below. Overall, 15 trials reported at least 1 outcome that showed statistically significant differences favouring the contracts group; six trials reported at least one outcome that showed statistically significant differences favouring the control group; and 26 trials reported at least 1 outcome without statistically significant differences between groups (see tables 2 to 5).
We present a narrative summary below for each of the health areas. Table 8; Table 9; Table 10; and Table 11 present all outcomes for each individual trial.
1. Addictions
Ten trials (in 12 reports) examined the effects of contracts in the context of substance addictions (Brockway 1977; Calsyn 1994; Curry 1988; Keane 1984; Lash 1998; O'Farrell 1984; Ossip‐Klein 1984; Piotrowski 1999; Poole 1981; Vinson 2000). See also Analyses 1.1 to 1.7, and Table 8.
Adherence
Adherence was measured in three different ways: (i) period of time abstinent (substance‐free samples); (ii) proportion of participants abstinent (substance‐free samples); and (iii) adherence to attending sessions (sensitisation sessions).
Substance abuse
(i) Period of time abstinent In one trial (Calsyn 1994), people in the contract group were abstinent for a longer period (as measured by positive urine analysis at 9 weeks post‐treatment) than people in the control group (result as reported by triallists; no extractable data).
In another trial (Piotrowski 1999), differences in abstinence duration were assessed at different time periods post treatment. No significant differences were found after 30 or 60 days of treatment; but significant differences favouring the intervention group were found in longer post‐treatment intervals, up to 180 days. For all participants (regardless the period of time they were on treatment) and individual substances, the only statistically significant differences reported were in the case of benzodiazepines and marijuana (favouring the intervention group). No differences were found for alcohol, amphetamines, barbiturates and cocaine .
(ii) Proportion of participants abstinent Calsyn 1994 reported the proportion of participants abstinent at 9 weeks and at 18 months, measured by urine analysis. At 9 weeks, the proportion of participants abstinent from opiates was significantly higher in the intervention group, but there were no differences between groups for cocaine. At 18 months, a significantly greater proportion of participants in the intervention group was abstinent compared with the control group: (a) regardless of the type of substance; (b) for cocaine, and; (c) for opiates. Detailed data was only reported for the 18‐month measurement point (see Analysis 1.1).
Analysis 1.1.
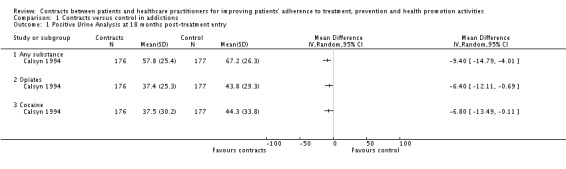
Comparison 1 Contracts versus control in addictions, Outcome 1 Positive Urine Analysis at 18 months post‐treatment entry.
In Piotrowski 1999 the proportion of participants in the contracts group that were abstinent after 120 days of treatment showed no difference with control group (measured by substance‐free samples). (See Analysis 1.2).
Analysis 1.2.
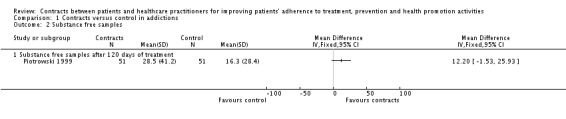
Comparison 1 Contracts versus control in addictions, Outcome 2 Substance free samples.
(iii) Adherence to attending sessions The percentage of participants present at sessions one to eight showed statistically significant differences favouring the contracts group for sessions one, three, four and six; but these differences vanished for sessions two, five, seven and eight (Ossip‐Klein 1984). In another trial, there were no statistically significant differences between groups in the number of participants who attended at least one aftercare group session nor in the mean number of aftercare sessions attended (Lash 1998). (See Analysis 1.5 and Analysis 1.6).
Analysis 1.5.
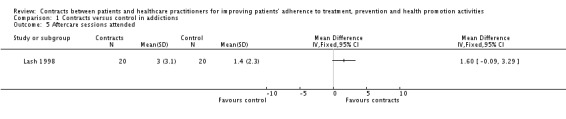
Comparison 1 Contracts versus control in addictions, Outcome 5 Aftercare sessions attended.
Analysis 1.6.
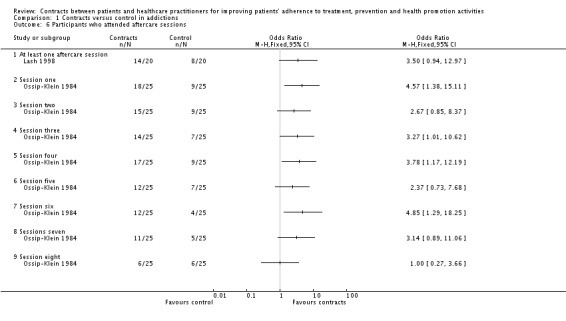
Comparison 1 Contracts versus control in addictions, Outcome 6 Participants who attended aftercare sessions.
Smoking
Contracts appeared to have little effect on participants' abstinence from smoking, when assessed in the included studies. (i) Period of time abstinent In one study (Poole 1981) the time remaining abstinent (measured by self‐reported daily cigarette consumption) was similar between groups (measured at any time period from 1 week to 12 months).
(ii) Proportion of participants abstinent In Curry 1988 the proportion of participants abstaining from smoking at any period (from treatment up to more than three months, measured by weekly self‐reported cigarette consumption) was also similar in both groups. In Brockway 1977 the participants in the contracts group smoked significantly fewer cigarettes (measured by individual self‐report) than people in the control group at 6 months follow up. However this difference vanished at 12 months follow up. In Poole 1981 there was no difference between participants in the control and contracts groups when cigarette consumption was compared with baseline smoking, from 1 week to 12 months follow up. (See Analysis 1.7).
Analysis 1.7.
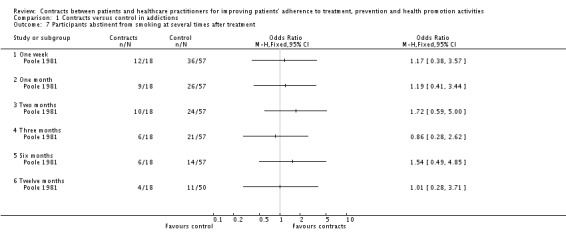
Comparison 1 Contracts versus control in addictions, Outcome 7 Participants abstinent from smoking at several times after treatment.
Secondary outcomes
There were no differences between groups in any of the trials in the following outcomes: dispensation of medication (Keane 1984), participants' satisfaction (O'Farrell 1984), change in Alcohol Use Disorder Identification Test (AUDIT, a score to screen for drinking problems) (Vinson 2000) and costs of treatments. (See Analysis 1.3).
Analysis 1.3.
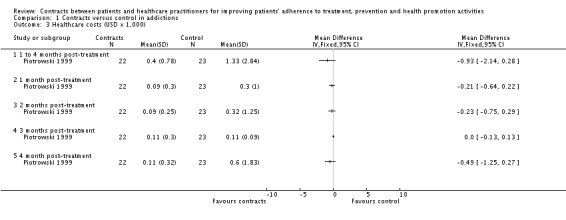
Comparison 1 Contracts versus control in addictions, Outcome 3 Healthcare costs (USD x 1,000).
O'Farrell 1984 measured participants' abilities to solve problems, and their perceptions about the treatment programmes, but the study did not report any statistical analysis nor enough data to be analysed post hoc.
A new outcome, related to the use of services, which was not foreseen at the protocol stage, is reported here. Contracts significantly increased the discharge rate of patients under methadone therapy (Calsyn 1994), because contingency contracting in this study included discharge for continuous positive urine analysis. In other words, contracts were unable to keep patients under treatment, however participants in the contracts group were statistically significantly less months out of treatment before readmission (i.e. they were readmitted more after a shorter period than participants in the control group).
2. Hypertension
Four trials examined the effects of contracts on a variety of outcomes, in the context of hypertension management (Binstock 1988; Hoelscher 1986;Schulman 1980;Swain 1981). (See also Table 9).
Adherence
Two trials reported adherence outcomes. Hoelscher 1986 examined the effects of contracts on relaxation practices. The 'group relaxation' (without contracts) group showed significantly better adherence to the relaxation practices than the control group, which itself showed better adherence than the 'group relaxation plus contract' group; that is, the group with contracts performed worst in terms of adherence. In another study (Swain 1981), however, fewer participants in the contracts group discontinued treatment, compared with the control group.
Secondary outcomes
Two of the four trials reported blood pressure changes. Binstock 1988 did not find any difference between groups at one year follow up. In Swain 1981, contracts statistically significantly improved the diastolic blood pressure measured over four visits (specific time periods not reported).
In Swain 1981, contracts significantly improved patients' knowledge about hypertension care issues. Participants' views on health care were examined in one trial (Schulman 1980) through the Active Patient Orientation scores reported by patients (health professionals support patients' motivations reinforcing their active participation, illness‐management is collaborative, clear instructions and skills training). Patients under contracts rated their care significantly higher in the Active Patient Orientation scores. In Hoelscher 1986, the cost‐effectiveness (improvement in blood pressure per hour of therapist contact) in the 'contracts plus group relaxation' group was significantly higher than in the 'individual relaxation' group.
3. Overweight
Three trials addressed contract interventions for overweight people (Aragona 1975; Craighead 1989; Murphy 1982). (See also Analysis 2.1; Analysis 2.2; and Table 10).
Analysis 2.1.
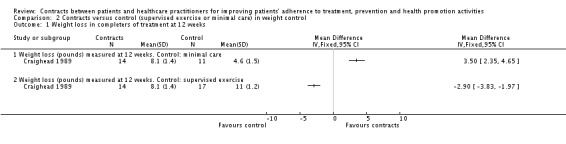
Comparison 2 Contracts versus control (supervised exercise or minimal care) in weight control, Outcome 1 Weight loss in completers of treatment at 12 weeks.
Analysis 2.2.
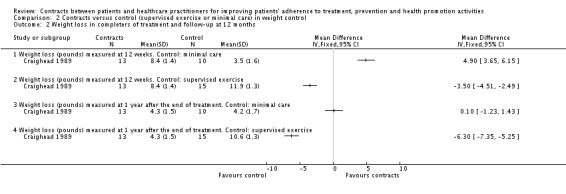
Comparison 2 Contracts versus control (supervised exercise or minimal care) in weight control, Outcome 2 Weight loss in completers of treatment and follow‐up at 12 months.
Adherence
None of the three trials reported adherence outcomes.
Secondary outcomes
In Aragona 1975 participants in the contracts group lost more weight than those in the control groups, both at the end of treatment (‐11.3 pounds in the intervention group compared with ‐9.5 and +0.5 pounds in the control groups), and at 8 weeks follow up (‐7.9 pounds in the intervention group compared with ‐5.0 and +3.6 pounds in the control groups).
In Craighead 1989 there were three groups: contracts, supervised exercise and minimal contact. Outcomes were measured at 12 weeks and 12 months. When data from the contracts and supervised exercise groups were pooled, people in these groups lost significantly more weight than those in the minimal care group. For those participants who completed the treatments, mean weight losses were respectively 8.1 pounds (contracts), 11 pounds (supervised exercise) and 4.6 pounds (minimal contact) (P < 0.05) (see Analysis 2.1). For longer term follow‐up (12 months), mean weight losses were 4.3 pounds (contracts), 10.6 pounds (supervised exercise) and 4.2 pounds (minimal contact) (P < 0.05). (See Analysis 2.2). Craighead 1989 also collected data on the self‐reported helpfulness of the treatment: for this outcome there were no statistically significant differences between the contracts group and the supervised exercise group.
In Murphy 1982 there were no statistically significant differences in any of the outcomes: mean weight loss, percentage of excess weight loss and weight reduction index.
4. Miscellaneous
Thirteen other studies covered a wide variety of health problems or areas, and were included in the miscellaneous category: Barrera 1977; Burkhart 2002; Claydon 1997; Flanders 1985; Haber 1993; Hammond 1999; Litzelman 1993; Mayer 1991; McLean 1973; Morgan 1988; Morisky 2001; Putnam 1994; Wurtele 1980. (See also Table 11).
Acne
Flanders 1985 looked at the effects of contingent and non‐contingent contracting on compliance with acne treatment and number of acne lesions. There was no difference in either of these outcomes between contract and control groups. (See also Table 11).
Acute bacterial infections
Putnam 1994 assessed the effects of 'self‐commitment' on the adherence to antibiotic treatment (score based on pill count) in patients suffering from acute bacterial infections. Adherence was significantly better in the 'self‐commitment' group than in the control group. There were no differences between groups, however, in self‐reported adherence, nor in the number of additional prescriptions required to finalise the treatment. (See Analysis 6.1 and Table 11).
Analysis 6.1.
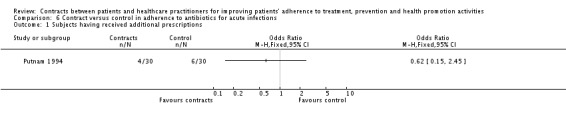
Comparison 6 Contract versus control in adherence to antibiotics for acute infections, Outcome 1 Subjects having received additional prescriptions.
Arthritis
Hammond 1999 examined the effects of a joint protection programme together with a contract on adherence to joint protection (Joint Protection Behaviour Assessment‐score measuring whether twenty routine daily life tasks are performed correctly in order not to cause joint damage) and to goals set in the joint protection programme (self‐reported joint protection homework), both showing statistically significant improvements in the intervention group. This effect was not observed in the second phase of the cross‐over trial. There were no differences between groups in knowledge or health‐related outcomes. (See also Table 11).
Asthma
A trial assessing a tripartite contractual approach (patients, practitioners and parents) for monitoring Peak Expiratory Flow rate (PEFR) in asthmatic children (Burkhart 2002) did not show any differences between groups in adherence to PEFR monitoring, nor in the number of asthma episodes. (See also Table 11).
Breast self examination
One trial (Mayer 1991) looked at the effects of contracts between female volunteers and healthcare practitioners on adherence to breast self examination. No differences were found between groups in relation to either the frequency of breast self examination, or the frequency of prompts by women's partners. (See also Table 11).
Contact lens care
Claydon 1997 examined the effects of a combined intervention consisting of contracts, teaching materials (posters, video) and reminders, on behaviours to take care of contact lenses, against routine care. There were no differences between groups in any of the targeted behaviours. (See also Table 11).
Depression
McLean 1973 evaluated the effects of contracts and training in social learning principles on changing patients and their partners' behaviours. Participants in the contract group, compared with those receiving routine care, showed significant improvement of targeted behaviours until 3 months follow up, as well as a decrease in negative reactions at the time the treatment ended. (See Analysis 5.1, and Table 11).
Analysis 5.1.
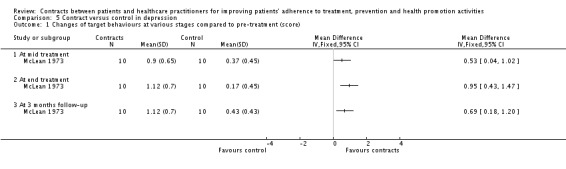
Comparison 5 Contract versus control in depression, Outcome 1 Changes of target behaviours at various stages compared to pre‐treatment (score).
Diabetes
Litzelman 1993 and Morgan 1988 examined the effects of contracts on the prevention of lower extremities abnormalities (musculoskeletal and dermatological) associated with diabetes, and on the treatment of type‐II diabetes, respectively. Outcomes in Litzelman 1993 included adherence outcomes (for example, washing the feet), health outcomes (for example, presence of foot lesions), and physician practice outcomes (for example, documentation of clinical observations). Some items in all three categories showed statistically significant improvements in the contracts groups (for example, reduction of serious foot lesions, of dry or cracked skin, washing the feet, inspecting the shoes), and in some other outcomes there were no differences between groups. (See Analysis 3.1). Knowledge of diabetes and its care statistically significantly improved in the control group (Morgan 1988), while in the same trial weight loss, reduction of fasting blood glucose and glycosylated haemoglobin were not statistically different between groups (the sample size, both groups combined, was 60. Knowledge was measured with the Diabetic Knowledge Scale (DIAKS), a 60‐item scale developed and tested for this study. (See also Table 11).
Analysis 3.1.
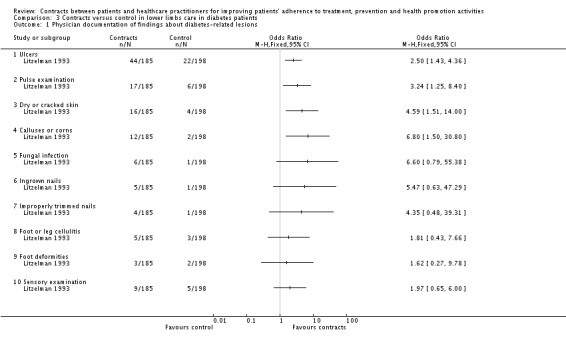
Comparison 3 Contracts versus control in lower limbs care in diabetes patients, Outcome 1 Physician documentation of findings about diabetes‐related lesions.
Phobia
The contracts intervention in Barrera 1977 aimed at reducing participants' phobia about snakes. The control group completed significantly more desensitisation sessions, and took more time to study the programme materials. At post‐test and follow‐up there was no benefit in any outcome compared with self‐administered systematic desensitisation. (See also Table 11).
Promotion of healthy diet and exercise
Another trial (Haber 1993) examined the effects of a combined intervention, including contracts, to reduce the amount of specific dietary components (and to improve other health behaviours such as exercise and stress management). The contracts group showed a statistically significant increase in fibre and decrease in salt intake, but showed no differences compared with the control group in intake of fats and sweets, and on the use of stress management techniques or practice of flexibility exercises. (See also Table 11).
Tuberculosis
Two trials related to tuberculosis adherence. One of them reported adherence to returning for the skin test reading (Wurtele 1980), which improved significantly in the intervention group. The other examined adherence to medication regimen (Morisky 2001) between four groups: contingency contracts, peer counseling, a combination of contracts and counseling, and usual care. Looking at differences between the contracts group and the other three, only a small difference significantly favouring the combination of contracts plus counseling group was found. (See also Analysis 4.1, and Table 11).
Analysis 4.1.
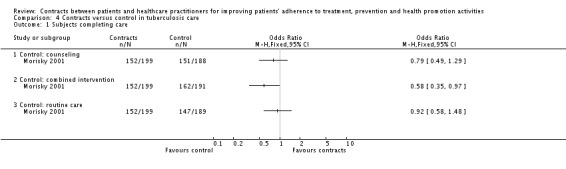
Comparison 4 Contracts versus control in tuberculosis care, Outcome 1 Subjects completing care.
None of the included studies reported any of the following outcomes: outcomes related to the contracts' contingencies, harms, or ethical issues.
Discussion
In this review we included 30 trials presented in 32 reports, the majority set in the USA and all of them in high income countries. The trials were undertaken in a range of settings (including some projects and services that were established especially for research purposes), and covered a wide range of health problems or areas, contract forms, participants, and outcomes.
Most of the trials were of poor design, or were poorly reported, or both. For example, only three trials reported their method of randomisation and only two mentioned a method of randomisation which allowed for the concealment of group allocation. Poor quality trials are more likely to be subject to bias and therefore the results are less reliable than those from better quality trials (Schulz 1995). In addition, the sample size of many trials was small. Over half the trials had more than two comparison groups, making group sample sizes even smaller. Small trials are more likely than larger trials to be insufficiently powered to detect statistically significant differences between groups.
In 25 of the included trials, the intervention groups involved in the contracting process also received other interventions intended to improve the measured outcomes. In addition, in 16 of the trials, 1 or more control groups received interventions other than routine care. It is therefore impossible, in most of the trials, to assess the effects of contracts per se compared to routine care; an assessment which would be very relevant for policy makers and consumers.
Contracts were described in varying degrees of detail, but they hardly met all assumptions as described by Quill (Quill 1983): terms and conditions explicitly stated; parties have unique responsibilities; the relationship between practitioners and patients is consensual, not obligatory; and all parties are able to negotiate. Furthermore, in the concordance paradigm (Jones 2003) contracts should not be simply understood as a way to engage patients to comply with a predefined set of instructions, but rather as a strategy to involve patients into a shared decision‐making process (Charles 1997). The requirements for shared decision making ‐ such as mechanisms for patients' preferences to be taken into account, information sharing and common decision on the regimens to follow ‐ were even more difficult to find in the included trials.
The great variety of health problems or areas, participants, interventions, control groups and outcomes precluded any attempt to pool data for meta‐analysis. The areas with the largest number of trials were those of substance addictions and hypertension. The data presented in the graphs has to be interpreted with caution, because we only included trials and outcomes with complete sets of data. Apart from one trial on adherence to antibiotic regimens for acute bacterial infections (Putnam 1994), all trials were related to chronic conditions.
Four of the seven trials dealing with alcohol or opiate addictions reported statistically significant differences in several outcomes favouring the contracts group. The findings in the review by Miller (Miller 2002) placed behavioural contracts as one of the top 10 (out of 46) treatment modalities for alcohol abuse (although important publication bias could not be ruled out in that review). However, some of those positive effects seen in our review were not consistent in all repeated measures over time. We could not identify any trial addressing the effects of opioid contracts in the management of opioids for the relief of chronic pain; contracts which are widely used but of doubtful efficacy (Fishman 1999).
In the area of smoking cessation (evaluated in three trials), our findings seem to agree with those in a review examining another behavioural intervention, namely competitions and incentives (Hey 2005): studies were underpowered and of variable quality. Furthermore, neither incentives, nor competitions, nor contracts, seemed to enhance long‐term cessation rates. In this review, the only positive effect reported (mean number of cigarettes smoked at several periods in time; Brockway 1977) vanished when measured at 12 months follow up.
All three trials about hypertension that reported blood pressure outcomes showed no differences between groups on blood pressure measurements (except for better diastolic blood pressure in the contracts group in Swain 1981). Adherence outcomes were both better (Swain 1981) and worse (Hoelscher 1986) in the contracts groups compared with the controls. Contracts in the context of hypertension seem relatively unexplored, despite the fact that in many countries blood pressure control falls far short of treatment goals and the recognised relevance of behavioural interventions to achieve those goals (Reunion 2006). The evidence from the included trials supporting the use of contracts for hypertension was very weak.
The external validity of the findings in the included trials is very limited, due to several factors: their narrow geographical scope; the settings which were specially established for research purposes in most cases; the ways that participants were recruited (for example, by advertisements); and the complexity and variety of contracts, co‐interventions and control group conditions, together with the inconsistent descriptions of those interventions. All these features discouraged any attempt to conduct a sub‐group analysis, since it would not be possible to control for each one of those factors. In many cases it is difficult, if not impossible, therefore, to attribute the effects seen to the impact of contracts alone. Furthermore, in many trials the selection criteria for participants were very stringent. It seems unlikely that the findings of these trials can be extrapolated to complex real situations as seen, for example, in young black men of deprived communities in whom depression, substance use (alcohol, tobacco and others), poor adherence and poor blood pressure outcomes have all been identified as related (Kim 2003).
There are some other critical factors to consider when deciding whether to introduce contracting within a healthcare delivery system. The included trials have addressed these factors little, if at all, namely: acceptability of contracts to healthcare practitioners; participants', patients' and carers' satisfaction; costs; clinicians' liability, perpetuation of stigma in patients (Fishman 2002b); and ethical considerations, especially where receiving treatment depends on patients adhering to the terms of the contract, or where financial rewards are used. Some of the outcomes listed in the protocol for this review addressed issues such as patients' participation in the contractual process, degree of shared decision making, harms or ethical issues; but none of the trials reported data on them. The lack of reporting on consumer participation highlights the provider‐centred approach, by which adherence is mainly seen as a patient's duty and practitioners remain in a patronising role; far from the concordance model. This may be partially due to the fact that most of the trials were conducted more than one decade ago. Future studies should also address the issue of harms. We saw in Hoelscher 1986 that the contracts group performed worse in adhering to relaxation practices. But contracts might also reduce the retention rate of patients, or affect the sincerity with which patients report events that may breach the terms of the contract.
Authors' conclusions
Contracts have been used as one among many other interventions for improving adherence.
Trials testing this intervention are generally small, and for many the quality is uncertain.
Some trials have demonstrated a positive effect of forming a contract in certain situations (for example, substance addiction), particularly when combined with other interventions, although it may be ineffective or harmful in other situations.
There is not enough evidence to recommend the widespread introduction of patient contracts into health services.
Existing small trials suggest that contracts may have a positive effect. This needs further evaluation with large, good quality randomised controlled trials to assess the effectiveness of patient contracts within established health systems. These should be:
designed to allow the effects of contracts and any co‐interventions to be assessed separately, as well as in combination where appropriate, taking into account the different features of contracts.
undertaken in health fields where adherence is particularly important or problematic, and where patients and/or carers think they may be valuable.
undertaken in a range of settings where they might be implemented if proven effective.
designed to assess potential harms.
Reports of these trials should use a standard definition of contract and describe the contract and contracting process in detail, including the practitioner‐patient relationship model and the extent of consumers' participation in the whole process.
Acknowledgements
We sincerely thank Dr Sophie Hill, Coordinating Editor of the Review Group, for her advice and support as contact editor of this review.
Many thanks to Ms Judy Stoelwinder and Ms Shirley Ward (former and current Trials Search Coordinators (respectively), Cochrane Consumers and Communication Review Group) for the elaboration of the search strategy and for providing extensive assistance with retrieval of studies, and to Ms Kelly Allen and Associate Professor Damien Jolley (Statistics Assistant and Statistics Editor, Consumers and Communication Review Group) for comprehensive checking of the data and analysis.
We thank the following people for their comments on the protocol from a consumer's perspective: Grindl Dockery, Community Research and Training Consultant (the Gender and Health group, Liverpool School of Tropical Medicine, Liverpool, UK); Kristin McCarthy (Director, Developing Patient Partnerships ‐formerly Doctor Patient Partnership, London, UK); Margaret Yarwood (Link Forum Support Network Coordinator), Renee Brenner (Aintree Hospital Trust and Patient Forum). We thank Dr. María Paz Loscertales who commented on the protocol and Dr. Aika Omari and Vittoria Lutje who provided technical guidance in the initial stages. Thanks also to Xavier Carbonell i Santías who assisted with an article in German and Kwang Shik Choi, in Korean.
Feedback on the protocol and review was received from three external referees (including one consumer) and five members of the editorial team of the Consumers and Communication Review Group.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 (contract or contracts or contracting).tw. 2 (agreement or agreements).tw. 3 (concord$ or negotiat$).tw. 4 (goal$ adj setting).tw. 5 or/1‐4 6 patient compliance/ 7 (compliance or comply or complying or complied).tw. 8 (adherence or adher or adhering or adhered).tw. 9 or/6‐8 10 5 and 9 11 exp patient care planning/ 12 (care plan$ or case plan$).tw. 13 case management.tw. 14 or/11‐13 15 5 and 14 16 exp decision making/ 17 (information adj3 shar$).tw. 18 exp professional patient relations/ 19 exp consumer participation/ 20 informed consent/ 21 partnership.tw. 22 or/16‐21 23 5 and 22 24 (behavioral adj3 contract$3).tw. 25 (behavioural adj3 contract$3).tw. 26 contingency contract$3.tw. 27 (contingent adj3 (contract$3 or intervention$ or reinforcement)).tw. 28 participation deposit$1.tw. 29 ((refund$or reward$ or incentive$ or penalt$ or punish$) adj5 contingent).tw. 30 ((refund$ or reward$ or incentive$ or penalt$ or punish$) adj5 (contract$ or agree$ or concord$)).tw. 31 monetary deposit.tw. 32 ((monetary or payment$ or voucher$ or token$) adj3 contingent).tw. 33 or/24‐32 34 10 or 15 or 23 or 33 35 randomized controlled trial.pt. 36 controlled clinical trial.pt. 37 randomized controlled trials.sh. 38 random allocation.sh. 39 double blind method.sh. 40 single blind method.sh. 41 or/35‐40 42 animals/ not (human/ and animal/) 43 41 not 42 44 clinical trial.pt. 45 exp clinical trials/ 46 (clin$ adj25 trial$).ti,ab. 47 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 48 placebos.sh 49 placebo$.ti,ab 50 random$.ti,ab. 51 research design.sh. 52 or/44‐51 53 52 not 42 54 43 or 53 55 34 and 54 56 cohort studies/ or cohort.tw. 57 (time adj series).tw. 58 (pre test or pretest or (post test or posttest)).tw. 59 or/56‐58 60 34 and 59 61 55 or 60
Data and analyses
Comparison 1.
Contracts versus control in addictions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Positive Urine Analysis at 18 months post‐treatment entry | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Any substance | 1 | Mean Difference (IV, Random, 95% CI) | Not estimable | |
| 1.2 Opiates | 1 | Mean Difference (IV, Random, 95% CI) | Not estimable | |
| 1.3 Cocaine | 1 | Mean Difference (IV, Random, 95% CI) | Not estimable | |
| 2 Substance free samples | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Substance free samples after 120 days of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 3 Healthcare costs (USD x 1,000) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 1 to 4 months post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 3.2 1 month post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 3.3 2 months post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 3.4 3 months post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 3.5 4 month post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4 Longest period of abstinence (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Continuous abstinence at 30 days post treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.2 Continuous abstinence at 60 days post treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.3 Continuous abstinence at 90 days post treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.4 Continuous abstinence at 120 days post treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.5 Continuous abstinence at 150 days post treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.6 Continuous abstinence at 180 days post treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.7 Substance free samples for alcohol | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.8 Substance free samples for amphetamines | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.9 Substance free samples for barbiturates | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.10 Substance free samples for benzodiazepines | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.11 Substance free samples for cocaine | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 4.12 Substance free samples for marijuana | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 5 Aftercare sessions attended | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Participants who attended aftercare sessions | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 At least one aftercare session | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 6.2 Session one | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 6.3 Session two | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 6.4 Session three | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 6.5 Session four | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 6.6 Session five | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 6.7 Session six | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 6.8 Sessions seven | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 6.9 Session eight | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 7 Participants abstinent from smoking at several times after treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 One week | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 7.2 One month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 7.3 Two months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 7.4 Three months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 7.5 Six months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 7.6 Twelve months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Analysis 1.4.
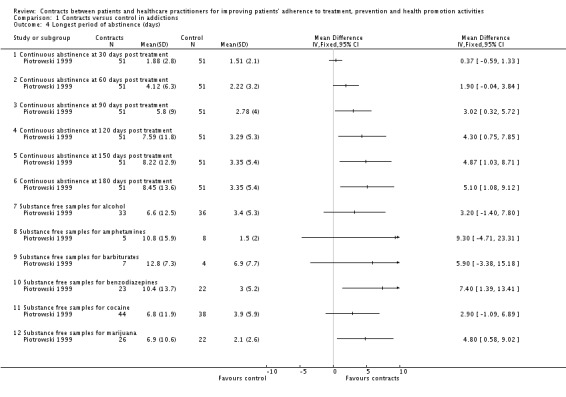
Comparison 1 Contracts versus control in addictions, Outcome 4 Longest period of abstinence (days).
Comparison 2.
Contracts versus control (supervised exercise or minimal care) in weight control
Comparison 3.
Contracts versus control in lower limbs care in diabetes patients
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physician documentation of findings about diabetes‐related lesions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Ulcers | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.2 Pulse examination | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.3 Dry or cracked skin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.4 Calluses or corns | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.5 Fungal infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.6 Ingrown nails | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.7 Improperly trimmed nails | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.8 Foot or leg cellulitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.9 Foot deformities | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.10 Sensory examination | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 4.
Contracts versus control in tuberculosis care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjects completing care | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Control: counseling | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.2 Control: combined intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| 1.3 Control: routine care | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 5.
Contract versus control in depression
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Changes of target behaviours at various stages compared to pre‐treatment (score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At mid treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 1.2 At end treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 1.3 At 3 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 6.
Contract versus control in adherence to antibiotics for acute infections
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjects having received additional prescriptions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
What's new
Last assessed as up‐to‐date: 28 May 2004.
| Date | Event | Description |
|---|---|---|
| 14 March 2009 | Amended | Correction of text formatting problem. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 29 July 2008 | Amended | We had used the trial authors' data where there was a discrepancy between that and the RevMan calculations. We have amended the review to present only the RevMan data, and this has resulted in minor changes to the results in relation to three included studies (Lash 1998, Morisky 2001 and Piotrowski 1999). After these amendments, overall fifteen (rather than sixteen) trials reported at least one outcome that showed statistically signficant differences favouring the contracts group, and six (rather than five) trials reported at least one outcome that showed differences favouring the control group. |
| 2 July 2008 | Amended | Converted to new review format. |
Differences between protocol and review
INCLUSION CRITERIA: the published protocol included quasi‐randomised trials, controlled before‐and‐after studies (CBAs) and interrupted time series (ITS) analyses. As more RCT's than expected were found on searching, we subsequently decided to include only RCTs.
OUTCOMES: We added "Utilisation of health services" in the review as this was found in one of the studies and seems relevant.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aragona 1975
| Methods | Randomised controlled trial with three groups. | |
| Participants | Country: USA. Setting: other. Health problem: overweight. Recruitment: health system and adverts. Participants: girls aged 5 to 11 who were overweight (n=15). | |
| Interventions | Contract features
Group 1: Contracts between parents and the providers of a weight loss programme. Parents gave a monetary deposit to the programme and received money back when their children achieved an agreed weight loss. Group 2: As per group 1, but parents also contracted to facilitate their child's weight loss by carrying out reinforcement techniques. Group 3: No contracts. |
|
| Outcomes | Mean weight change (pounds) from start of treatment to end of treatment, and to 8 week follow‐up. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Barrera 1977
| Methods | Randomised controlled trial with three groups. | |
| Participants | Country: USA. Setting: NA. Health problem: snake phobia. Recruitment: adverts. Participants: adults (n=24). | |
| Interventions | Contract features:
Group 1: Self administered desensitisation workbook. Group 2: Self administered desensitisation workbook with contract to reward self for completion of workbook. Group 3: Placebo. |
|
| Outcomes | Number of desensitisation sessions attended; time spent studying materials. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Binstock 1988
| Methods | Randomised controlled trial with five different groups; two with contracts and three without. | |
| Participants | Country: USA. Setting: PHC. Health problem: hypertension. Recruitment: health system. Participants: adults (n=112). | |
| Interventions | Contract features:
Group 1: Self‐reward compliance contracts + educational program. Group 2: Self‐reward compliance contracts + educational programme + BP measurement at home + calendar pills. Group 3: Bi‐monthly educational program. Group 4: Educational + BP measurement at home. Group 5: Calendar pills. |
|
| Outcomes | Change of blood pressure from baseline to 1 year follow up. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Brockway 1977
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: speciality. Health problem: addictions (smoking). Recruitment: adverts. Participants: adults (n=27). | |
| Interventions | Contract features:
Experimental group: smoking cessation programme including contingency contracting (return of deposit based on attendance at meetings and completion of assignments). Control group: waiting list. |
|
| Outcomes | Mean number of cigarettes smoked. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Burkhart 2002
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: NA. Health problem: asthma. Recruitment: health system. Participants: children (n=42). | |
| Interventions | Contract features:
Group 1: Contingency management (child contracted with parents and investigator to record daily peak expiratory flow rate (PEFR)). Group 2: Usual care. |
|
| Outcomes | Adherence to PEFR monitoring over a 5 week period. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Calsyn 1994
| Methods | Randomised controlled trial with six groups. | |
| Participants | Country: USA. Setting: NA. Health problem: addictions (opiate). Recruitment: health system. Participants: adult patients (n=353). | |
| Interventions | Contract features:
Three of the groups (Groups 1 to 3) included contingency contracting ‐ treatment depending on reaching goals for abstinence from illicit drugs. Group 1: Medication only: saw counsellor to complete standard treatment. Group 2: Standard: counselling sessions and optional drug education classes. Group 3: Enhanced: as per Group 2 plus relapse prevention skill training group and weekly group treatment Groups 4, 5 and 6 mirrored the above groups but without the use of contingency contracts. |
|
| Outcomes | Rates of illicit drug and alcohol use, discharge rates and length of time to readmission for those discharged. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Claydon 1997
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: UK Setting: speciality. Health problem: contact lenses. Recruitment: NA. Participants: contact lens wearers (n=80). | |
| Interventions | Contract features:
Group 1: Teaching programme on contact lens care, including contract to sign. Group 2: Usual care. All participants received a free supply of contact lenses for a year. |
|
| Outcomes | Self reported contact lens care behaviours. | |
| Notes | Participants were unaware of being in a trial. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Craighead 1989
| Methods | Randomised controlled trial with three groups. | |
| Participants | Country: USA Setting: other. Health problem: overweight. Recruitment: adverts. Participants: women aged 18 to 30 and 15 to 45 pounds overweight (n=62). | |
| Interventions | Contract features:
Group 1: Contracted exercise and written lessons. Group 2: Instructions and supervised exercise. Group 3: Instructions and minimal contact. |
|
| Outcomes | Weight loss and Harvard Step Test fitness score at follow‐up. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Curry 1988
| Methods | Randomised controlled trial with four groups. | |
| Participants | Country: USA. Setting: other. Health problem: addictions (smoking). Recruitment: adverts. Participants: adult smokers (n=139). | |
| Interventions | Contract features:
Two different smoking cessation programmes, one of which included contingency contracting. Both programmes were subdivided into self‐help and group support groups. Participants with contracts contracted to send $15 to a person or organisation they disliked if they smoked after their quit date. |
|
| Outcomes | Abstinence rates at 3, 6 and 9 months after treatment. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Flanders 1985
| Methods | Randomised controlled trial with four groups. | |
| Participants | Country: USA. Setting: NA. Health problem: acne. Recruitment: other (screened as part of a larger study). Participants: college students (n=42). | |
| Interventions | Contract features:
Group 1: Non‐contingent contract (agreement to return self‐monitoring cards) with education and self monitoring medication card. Group 2: Contingent contract (agreement to return self‐monitoring cards with chance to win prizes for each returned) with education and self monitoring medication card. Group 3: Education and self‐monitoring card. Group 4: Waiting list. |
|
| Outcomes | Acne cream compliance rate and number of acne lesions. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Haber 1993
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: speciality. Health problem: healthy diet. Recruitment: adverts. Participants: adults over the age of 55 (n=64). | |
| Interventions | Contract features:
Experimental group: health education sessions plus peer group support sessions where behaviour changes were agreed through group discussion and participants signed a contract to undertake these changes. Control group: received health education classes only. |
|
| Outcomes | Change in consumption of salt, sweets, fat and fibre. Practising of relaxation techniques and body movements. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Hammond 1999
| Methods | Randomised controlled cross‐over trial. | |
| Participants | Country: USA. Setting: NA. Health problem: arthritis. Recruitment: health system. Participants: adults (n=35). | |
| Interventions | Contract features:
Experimental group: teaching joint protection techniques, including contracting as part of a goal‐setting and self‐monitoring process, compared with no intervention. Control group: later received the same intervention. |
|
| Outcomes | Use of joint protection techniques at 12 and 24 weeks. Measures of pain, functional disability, grip strength, self‐efficacy and helplessness. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Hoelscher 1986
| Methods | Randomised controlled trial with four groups, one of the groups using contracts. | |
| Participants | Country: USA. Setting: NA. Health problem: hypertension. Recruitment: adverts. Participants: adults (n=50). | |
| Interventions | Contract features:
Group 1: Group relaxation training plus contingency contracting. The contract specified daily or weekly consequences to be given by the participant's spouse for practicing relaxation exercises. Group 2: Individual relaxation training. Group 3: Group relaxation training. Group 4: Waiting list. |
|
| Outcomes | Compliance with relaxation exercises and changes in blood pressure at week 5 to 6 and week 9 to 10. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Keane 1984
| Methods | Randomised controlled trial with three groups. | |
| Participants | Country: USA. Setting: Hospital (Veterans Administration Medical Center ‐ Alcohol Dependence Treatment Program). Health problem: addictions (alcohol). Recruitment: health system. Participants: men (n=25). | |
| Interventions | Contract features:
Group 1: Contracting and recording ‐ patients took their daily medication in front of a significant other and they both recorded, signed and dated it on a standard form. Group 2: Contracting and recording plus significant other given instructions for reinforcement. Group 3: Explanations in relation to disulfiram (Antabuse); phone calls to check on use and aid in resolving difficulties. |
|
| Outcomes | Participants who collected monthly prescriptions for disulfiram (Antabuse) for 3 months. Participants whose significant other reported disulfiram being taken daily at 3 months, percentage of aftercare sessions attended. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Lash 1998
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: speciality (Veterans Affairs Medical Center inpatient substance abuse treatment program). Health problem: addictions (alcohol and drugs). Recruitment: health system. Participants: adults (n=40). | |
| Interventions | Contract features:
Experimental group: aftercare orientation session plus aftercare participation contract. Control group: videotape of motivational speaker on aftercare. |
|
| Outcomes | Number of aftercare sessions attended. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Litzelman 1993
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: PHC. Health problem: diabetes. Recruitment: health system. Participants: adults (n=395). | |
| Interventions | Contract features:
Experimental group: education sessions, individually‐negotiates foot care contracts and postal reminders about foot care. Control group: routine care. |
|
| Outcomes | Foot lesions at 1 year, and various foot care behaviours. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Mayer 1991
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: other. Health problem: breast self‐examination. Recruitment: adverts. Participants: female University employees (n=36). | |
| Interventions | Contract features:
Experimental group: contract to remind to perform breast self‐examination. Control group: no contracting. |
|
| Outcomes | Frequency of breast self‐examination, and frequency of being prompted. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
McLean 1973
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: Canada. Setting: other. Health problem: depression. Recruitment: health system. Participants: adults aged 20‐55 and their spouses (n=20). | |
| Interventions | Contract features:
Experimental group: contract between husband and wife relating to the communication between themselves, training in social learning principles, and course in immediate feedback. Control group: usual care and monitoring the course of depression. |
|
| Outcomes | Target communication behaviours and negative reactions. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Morgan 1988
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: speciality. Health problem: diabetes. Recruitment: health system and adverts. Participants: adults (n=60). | |
| Interventions | Contract features:
Experimental group: educational programme on the management of diabetes, with weekly contracts for behaviour change in exchange for reinforcers such as flowers or lottery tickets. Control group: similar education programme without contracts. |
|
| Outcomes | Change in weight, fasting blood glucose, glycosylated haemoglobin and knowledge score at week 8. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Morisky 2001
| Methods | Randomised controlled trial with four groups. | |
| Participants | Country: USA. Setting: PHC. Health problem: tuberculosis. Recruitment: health system. Participants: adolescents (n=794) and their parents. | |
| Interventions | Contract features:
Group 1: Contingency contracts negotiated between adolescents and their parents where the parent provide an incentive in return for adolescent adhering to prescribed medication. Group 2: Contingency contracts plus peer counselling. Group 3: Peer counselling only. Group 4: Routine care. |
|
| Outcomes | Completion of treatment. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Murphy 1982
| Methods | Randomised controlled trial with a 2 x 2 factorial design plus 2 control groups. | |
| Participants | Country: USA. Setting: other. Health problem: overweight. Recruitment: adverts. Participants: adults (n=97 couples). | |
| Interventions | Contract features:
Group 1: Attended weight loss education sessions alone and made contingency contracts selecting their own punishments and rewards for specified weight loss behaviours. Group 2: Attended weight loss education sessions alone and made contingency contracts as for group 1 but agreed and signed by both themselves and their spouse. Group 3: Attended weight loss education sessions with their spouse and made contingency contracts selecting their own punishments and rewards for specified weight loss behaviours. Group 4: Attended weight loss education sessions alone and made contingency contracts agreed and signed by both themselves and their spouse. Group 5: Attendance at a weight‐loss support group. Group 6: No intervention. |
|
| Outcomes | Mean weight loss, percentage excess weight loss, and weight reduction index at 10 weeks. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
O'Farrell 1984
| Methods | Randomised controlled trial with three groups. | |
| Participants | Country: USA. Setting: other. Health problem: addictions (alcohol). Recruitment: health system. Participants: men (n=36) and their wives. | |
| Interventions | Contract features:
Group 1: Contract: The husband agrees to take disulfiram (Antabuse) daily and the wife observes and records it. In return she agrees not to mention any past drinking or any fears about future drinking. Couple counselling stressing goodwill and caring behaviours. Group 2: Couple counselling with catharsis, ventilation, sharing of feelings. Group 3: No marital treatment. |
|
| Outcomes | Satisfaction with the programme, ability to solve problems and adherence to sessions. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Ossip‐Klein 1984
| Methods | Cluster randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: other. Health problem: addictions (alcohol). Recruitment: health system. Participants: adult male (n=50). | |
| Interventions | Contract features:
Experimental group: contract with a significant other or self, agreeing to post a prompt calendar in a prominent place, attend aftercare sessions and telephone at least an hour in advance if unable to attend aftercare. Control group: no contracts or prompt calendars. |
|
| Outcomes | Attendance at aftercare sessions. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Yes | A ‐ Adequate |
Piotrowski 1999
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: speciality. Health problem: addictions (opiate). Recruitment: other. Participants: adults (n=102). | |
| Interventions | Contract features:
Experimental group: contracts using monetary (in the form of tokens) rewards for abstinence from illicit drugs and alcohol as assessed in random tests. Control group: random tests and feedback only. |
|
| Outcomes | Number of substance free samples and longest period of abstinence at different follow up times. Costs. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Poole 1981
| Methods | Randomised controlled trial with four groups. | |
| Participants | Country: Australia. Setting: NA. Health problem: addictions (smoking). Recruitment: adverts. Participants: adults under the age of 50 (n=75). | |
| Interventions | Contract features.
Group 1: Rapid smoking sessions. Group 2: Rapid smoking sessions plus relaxation training. Group 3: Rapid smoking, relaxation and contingency contracting; drawn up between patient and significant other to reinforce patients' not smoking. Group 4: Contingent rapid smoking; patients who smoked were required to attend extra rapid smoking sessions. |
|
| Outcomes | Abstinence from smoking from 1 week to 12 months after treatment. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Putnam 1994
| Methods | Randomised controlled trial with two groups. | |
| Participants | Country: USA. Setting: other. Health problem: acute infections. Recruitment: health system. Participants: students aged 18‐26 (n=110). | |
| Interventions | Contract features:
Experimental group: patients signed commitment to take all their medication. Control group: usual care. |
|
| Outcomes | Adherence based on pill counts, self‐reported adherence and additional prescriptions received. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Schulman 1980
| Methods | Randomised controlled trial with three groups. | |
| Participants | Country: USA. Setting: hospital. Health problem: hypertension. Recruitment: health system. Participants: adults (n=105). | |
| Interventions | Contract features:
Group 1: Education booklet plus contingency contracts with behavioural goals; patients received an agreed reward from a nurse for certain behaviours. Group 2: Education booklet. Group 3: Usual care only. |
|
| Outcomes | Active patient orientation score, indices of resources score and facts index. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Swain 1981
| Methods | Randomised controlled trial with three groups. | |
| Participants | Country: USA. Setting: PHC. Health problem: hypertension. Recruitment: health system. Participants: adults (n=115). | |
| Interventions | Contract features:
Group 1: Education booklet plus contingency contracts with behavioural goals; patients received an agreed reward from a nurse for reaching agreed goals. Group 2: Education booklet. Group 3: Usual care only. |
|
| Outcomes | Change in knowledge score, number of participants discontinuing treatment, diastolic blood pressure. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Vinson 2000
| Methods | Randomised controlled trial. | |
| Participants | Country: USA. Setting: PHC. Health problem: addictions (alcohol). Recruitment: health system. Participants: adult patients (n=80). | |
| Interventions | Contract features:
Experimental group: contract for changing drinking behaviour produced using options within a computer programme, reviewed by a physician and signed by both the physician and the patient. Control group: screening and baseline assessment. |
|
| Outcomes | Alcohol Use Disorders Identification Test (AUDIT) and Addiction Severity Index (ASI) scores at 12 months. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Yes | A ‐ Adequate |
Wurtele 1980
| Methods | Randomised controlled trial with three groups. | |
| Participants | Country: USA. Setting: other. Health problem: tuberculosis. Recruitment: adverts. Participants: students (n=1946). | |
| Interventions | Contract features:
Group 1: participants were asked for both verbal and written commitment to return. Group 2: participants were asked for their verbal commitment to return for skin‐test reading in 48 hours. Group 3: participants were told to return to have skin test read 48 hours later. |
|
| Outcomes | Number attending for skin test reading. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
NA: information not available; PHC: Primary Health Care.
The number of participants reflects the number entering the studies, which may differ from the number analysed.
Parties are categorised as healthcare practitioners, participants/patients, and carers (including peers and significant others). Tripartite contracts involve patients, carers and healthcare practitioners.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Azrin 1994 | Not an RCT |
| Becona 1997 | Not an RCT |
| Bishai 2003 | Not an RCT |
| Black 1983 | Compares two types of contracts |
| Bowers 1987 | Compares two types of contracts |
| Brubaker 2003 | Not an RCT |
| Budney 2001 | No comparison group |
| Bull 2000 | Not an RCT |
| Calsyn 1996 | Does not assess the effects of contracts |
| Capelli 1990 | Not an RCT (see notes) |
| Christensen 1995 | Not an RCT |
| Coelho 1985 | No data on outcomes comparing intervention and control |
| Cottler 1998 | Not an RCT. Not a contract intervention |
| Cummings 1981 | Not an RCT |
| Davis 1995 | Compares two types of contracts |
| Donaldson 1997 | Not an RCT |
| Epstein 2001 | Not a contract intervention |
| Feeney 2001 | Not an RCT |
| Feeney 2002 | Not an RCT |
| Fleming 1997 | Not a contract intervention |
| Hamilton 1993 | Not a contract intervention |
| Harzer 2000 | Not an RCT |
| Hennig 1998 | Not a contract intervention. No appropriate outcomes |
| Jeffery 1983 | Compares two types of contracts; no control group |
| Jeffery 1984 | Not an RCT (same study as Jeffery 1983) |
| Jeffrey 1975 | Not a contract intervention |
| Johnson 1991 | Not an RCT |
| Jones 1993 | Not an RCT |
| Kim 1991 | Not an RCT |
| Laidlaw 1999 | Not an RCT |
| Leslie 1991 | Not an RCT |
| Lierman 1994 | No data on outcomes comparing contracts with control |
| Lowe 1997 | No appropriate outcomes |
| Messina 2003 | Not a contract intervention |
| Miller 1995 | Not an RCT |
| Napolitan 1999 | Not an RCT |
| Neale 1991 | Not an RCT |
| Neuberger 1993 | Not an RCT |
| Norton 1980 | Not a contract intervention, no appropriate outcomes |
| Ordman 1985 | Not a contract intervention |
| Pantalon 2001 | Not an RCT |
| Paxton 1980 | Not an RCT |
| Radojevic 1992 | Not an RCT |
| Resnicow 1997 | Only data on outcomes for the intervention group |
| Sagawa 2003 | Not an RCT |
| Sand 1974 | No data on outcomes |
| Saxon 1993 | Not an RCT |
| Schinke 1976 | Not a contract intervention |
| Solanto 1994 | Comparing two types of contracts |
| Stuart 1976 | Not a health related topic |
| Toseland 1983 | Not an RCT |
| Tusel 1994 | Not enough data on outcomes, no response to attempted contact with author(s) |
| Ureda 1980 | Two types of contracts |
| Van Dover 1985 | Not enough data on outcomes, no response to attempted contact with author(s) |
| Villano 2002 | Not an RCT |
| Wysocki 1989 | Not an RCT |
| Zandee 1996 | Not an RCT |
For additional information about the exclusion of studies other than RCTs, see the 'Notes' section.
Contributions of authors
PG and XBC conceptualised the review.
XBC wrote the first drafts of the protocol, and both made changes to the protocol in response to editors' and external peer‐reviewers' comments.
XBC and KA worked through all stages of the review. MP contributed to the later drafts of the review. PG participated in applying the inclusion criteria to some studies, resolved disagreements and reviewed the process.
Sources of support
Internal sources
No sources of support supplied
External sources
The Department of Health, UK.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
- Aragona J, Cassady J, Drabman RS. Treating overweight children through parental training and contingency contracting. Journal of Applied Behavior Analysis 1975;8(3):269‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera M, Jr Rosen GM. Detrimental effects of a self‐reward contracting program on subjects' involvement in self‐administered desensitization. Journal of Consulting and Clinical Psychology 1977;45(6):1180‐1. [DOI] [PubMed] [Google Scholar]
- Binstock ML, Franklin KL. A comparison of compliance techniques on the control of high blood pressure. American Journal of Hypertension 1988;1(3 Pt 3):192S‐4S. [DOI] [PubMed] [Google Scholar]
- Brockway BS, Kleinmann G, Edleson J, Grunewald K. Non‐aversive procedures and their effect on cigarette smoking. Addictive Behaviors 1977;2(Sup 3):121‐8. [DOI] [PubMed] [Google Scholar]
- Burkhart PV, Dunbar‐Jacob JM, Fireman P, Rohay J. Children's adherence to recommended asthma self‐management. Pediatric Nursing 2002;28(4):409‐14. [PubMed] [Google Scholar]
- Calsyn DA, Wells EA, Saxon AJ, Jackson TR, Wrede AF, Stanton V, Fleming C. Contingency management of urinalysis results and intensity of counseling services have an interactive impact on methadone maintenance treatment outcome. Journal of Addictive Diseases 1994;13(3):47‐63. [DOI] [PubMed] [Google Scholar]; Saxon AJ, Wells EA, Fleming C, Jackson TR, Calsyn DA. Pre‐treatment characteristics, program philosophy and level of ancillary services as predictors of methadone maintenance treatment outcome. Addiction 1996;91(8):1197‐209. [DOI] [PubMed] [Google Scholar]
- Claydon BE, Efron N, Woods C. A prospective study of the effect of education on non‐compliant behaviour in contact lens wear. Ophthalmic & Physiological Optics 1997;17(2):137‐46. [PubMed] [Google Scholar]
- Craighead LW. Supervised exercise in behavioral treatment for moderate obesity. Behavior Therapy 1989;20(1):49‐59. [Google Scholar]
- Curry SJ, Marlatt GA, Gordon J, Baer JS. A comparison of alternative theoretical approaches to smoking cessation and relapse. Health Psychology 1988;7(6):545‐56. [DOI] [PubMed] [Google Scholar]
- Flanders PA, McNamara JR. Enhancing acne medication compliance: a comparison of strategies. Behaviour Research & Therapy 1985;23(2):225‐7. [DOI] [PubMed] [Google Scholar]
- Haber D, Lacy MG. Evaluation of a socio‐behavioral intervention for changing health behaviors of older adults. Behavior, Health and Aging 1993;3(2):73‐85. [Google Scholar]
- Hammond A, Lincoln N, Sutcliffe L. A crossover trial evaluating an educational‐behavioural joint protection programme for people with rheumatoid arthritis. Patient Education & Counseling 1999;37(1):19‐32. [DOI] [PubMed] [Google Scholar]
- Hoelscher TJ, Lichstein KL, Rosenthal TL. Home relaxation practice in hypertension treatment: Objective assessment and compliance induction. Journal of Consulting & Clinical Psychology 1986;54(2):217‐21. [DOI] [PubMed] [Google Scholar]
- Keane TM, Foy DW, Nunn B, Rychtarik RG. Spouse contracting to increase antabuse compliance in alcoholic veterans. Journal of Clinical Psychology 1984;40(1):340‐4. [DOI] [PubMed] [Google Scholar]
- Lash SJ. Increasing participation in substance abuse aftercare treatment. American Journal of Drug & Alcohol Abuse 1998;24(1):31‐6. [DOI] [PubMed] [Google Scholar]
- Litzelman DK, Slemenda CW, Langefeld CD, Hays LM, Welch MA, Bild DE, et al. Reduction of lower extremity clinical abnormalities in patients with non‐insulin‐dependent diabetes mellitus. A randomized, controlled trial. Annals of Internal Medicine 1993;119(1):36‐41. [DOI] [PubMed] [Google Scholar]
- Mayer JA, Beach DL, Hillman E, Kellogg MC, Carter M. The effects of co‐worker‐delivered prompts on breast self‐examination frequency. American Journal of Preventive Medicine. 1991;7(1):9‐11. [PubMed] [Google Scholar]
- McLean PD, Ogston K, Grauer L. A behavioral approach to the treatment of depression. Journal of Behavior Therapy & Experimental Psychiatry 1973;4(4):323‐30. [Google Scholar]
- Morgan BS, Littell DH. A closer look at teaching and contingency contracting with type II diabetes. Patient Education and Counseling 1988;12(2):145‐58. [Google Scholar]
- Morisky DE, Malotte CK, Ebin V, Davidson P, Cabrera D, Trout PT, Coly A. Behavioral interventions for the control of tuberculosis among adolescents. Public Health Reports 2001;116(6):568‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JK, Williamson DA, Buxton AE, Moody SC, Absher N, Warner M. The long‐term effects of spouse involvement upon weight loss and maintenance. Behavior Therapy 1982;13(5):681‐93. [Google Scholar]
- O'Farrell TJ, Cutter HSG. Behavioral marital therapy for male alcoholics: clinical procedures from a treatment outcome study in progress. American Journal of Family Therapy 1984;13(3):33‐46. [Google Scholar]
- Ossip‐Klein DJ, Vanlandingham W, Prue DM, Rychtarik RG. Increasing attendance at alcohol aftercare using calendar prompts and home based contracting. Addictive Behaviors. 1984;9(1):85‐9. [DOI] [PubMed] [Google Scholar]
- Hartz DT, Meek P, Piotrowski NA, Tusel DJ, Henke CJ, Delucchi K, et al. A cost‐effectiveness and cost‐benefit analysis of contingency contracting‐enhanced methadone detoxification treatment. American Journal of Drug & Alcohol Abuse 1999;25(2):207‐18. [DOI] [PubMed] [Google Scholar]; Piotrowski NA, Tusel DJ, Sees KL, Reilly PM, Banys P, Meek P, Hall SM. Contingency contracting with monetary reinforcers for abstinence from multiple drugs in a methadone program. Experimental & Clinical Psychopharmacology 1999;7(4):399‐411. [DOI] [PubMed] [Google Scholar]
- Poole AD, Sanson‐Fisher RW, German GA. The rapid‐smoking technique: therapeutic effectiveness. Behaviour Research & Therapy 1981;19(5):389‐97. [DOI] [PubMed] [Google Scholar]
- Putnam DE, Finney JW, Barkley PL, Bonner MJ. Enhancing commitment improves adherence to a medical regimen. Journal of Consulting & Clinical Psychology 1994;62(1):191‐4. [DOI] [PubMed] [Google Scholar]
- Schulman BA, Swain MA. Active patient orientation. Patient Counselling and Health Education 1980;2(1):32‐7. [DOI] [PubMed] [Google Scholar]
- Swain MA, Steckel SB. Influencing adherence among hypertensives. Research in Nursing & Health 1981;4(1):213‐22. [DOI] [PubMed] [Google Scholar]
- Vinson DC, Devera‐Sales A. Computer‐generated written behavioral contracts with problem drinkers in primary medical care. Substance Abuse 2000;21(4):215‐22. [DOI] [PubMed] [Google Scholar]
- Wurtele SK, Galanos AN, Roberts MC. Increasing return compliance in a tuberculosis detection drive. Journal of Behavioral Medicine 1980;3(3):311‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Azrin NH, McMahon PT, Donohue B, Besalel VA, Lapinski KJ, Kogan ES, et al. Behavior therapy for drug abuse: a controlled treatment outcome study. Behaviour Research & Therapy 1994;32(8):857‐66. [DOI] [PubMed] [Google Scholar]
- Becona E, Vazquez FL. Does using relapse prevention increase the efficacy of a program for smoking cessation?: an empirical study. Psychological Reports 1997;81(1):291‐6. [DOI] [PubMed] [Google Scholar]
- Bishai D, Qureshi A, Cantu N, Parks C. Contracting with children and helmet distribution in the emergency department to improve bicycle helmet use. Academic Emergency Medicine 2003;10(12):1371‐7. [DOI] [PubMed] [Google Scholar]
- Black DR, Scherba DS. Contracting to problem solve versus contracting to practice behavioral weight loss skills. Behavior Therapy 1983;14(1):100‐9. [Google Scholar]
- Bowers TG, Winett RA, Frederiksen LW. Nicotine fading, behavioral contracting, and extended treatment: effects on smoking cessation. Addictive Behaviors 1987;12(2):181‐4. [DOI] [PubMed] [Google Scholar]
- Brubaker DA, Leddy JJ. Behavioral contracting in the treatment of eating disorders. Physician & Sportsmedicine 2003;31(9):2003. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha H. Abstinence‐based vouchers delivered without psychotherapy increase abstinence during treatment for marijuana dependence. Drug and Alcohol Dependence 2001;63(Suppl 1):21. [Google Scholar]
- Bull MJ, Hansen HE, Gross CR. A professional‐patient partnership model of discharge planning with elders hospitalized with heart failure. Applied Nursing Research 2000;13(1):19‐28. [DOI] [PubMed] [Google Scholar]
- Calsyn DA, Wells EA, Saxon AJ, Jackson TR, Stanton VV. Outcome of a second episode of methadone maintenance. Drug & Alcohol Dependence 1996;43(3):163‐8. [DOI] [PubMed] [Google Scholar]
- Capelli CA. Behavioral contracting with a monetary incentive program to improve fluid compliance of hemodialysis patients [PhD thesis]. Hattiesburg (MS): The University of Southern Mississippi, 1990. [Google Scholar]
- Christensen MH. The effects of support, adherence, and behaviors chosen in contingency contracts on metabolic outcomes in persons with Type II diabetes mellitus [PhD thesis]. Ann Arbor (MI): The University of Michigan, 1995. [Google Scholar]
- Coelho RJ. Longest prior abstinence and cessation of smoking. Psychological Reports 1985;56(2):468‐70. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Leukefeld C, Hoffman J, Desmond D, Wechsberg W, Inciardi JA, et al. Effectiveness of HIV risk reduction initiatives among out‐of‐treatment non‐injection drug users. Journal of Psychoactive Drugs 1998;30(3):279‐90. [DOI] [PubMed] [Google Scholar]
- Cummings KM, Becker MH, Kirscht JP, Levin NW. Intervention strategies to improve compliance with medical regimens by ambulatory hemodialysis patients. Journal of Behavioral Medicine 1981;4(1):111‐27. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Baker LJV. Smoking cessation: the use of a choice of strategies to aid cessation and maintenance. Irish Journal of Psychology 1995;16(2):150‐61. [Google Scholar]
- Donaldson D, Spirito A, Arrigan M, Weiner, Aspel J. Structured disposition planning for adolescent suicide attempters in a general hospital: preliminary findings on short‐term outcome. Archives of Suicide Research 1997;3(4):271‐82. [Google Scholar]
- Epstein DH, Hawkins W, Umbricht S, Preston KL. Contingency management and cognitive‐behavioral therapy to reduce cocaine use:emergent effect 1 year later?. Drug and Alcohol Dependence 2001;63(Suppl 1):43‐4. [Google Scholar]
- Feeney GFX, Young RMcD, Connor JP, Tucker J, McPherson A. Outpatient cognitive behavioural therapy programme for alcohol dependence: impact of naltrexone use on outcome. Australian & New Zealand Journal of Psychiatry 2001;35(4):443‐8. [DOI] [PubMed] [Google Scholar]
- Feeney GF, Young RM, Connor JP, Tucker J, McPherson A. Cognitive behavioural therapy combined with the relapse‐prevention medication acamprosate: are short‐term treatment outcomes for alcohol dependence improved?. Australian & New Zealand Journal of Psychiatry 2002;36(5):622‐8. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community‐based primary care practices. JAMA 1997;277(13):1039‐45. [PubMed] [Google Scholar]
- Hamilton GA, Roberts SJ, Johnson JM, Tropp JR, Anthony‐Odgren D, Johnson BF. Increasing adherence in patients with primary hypertension: an intervention. Health Values 1993;17(1):3‐11. [Google Scholar]
- Harzer W, Karmann A. Long term compliance of patients ‐ can we stimulate it financially? A result from orthodontics [Lässt sich die Patientencompliance nachhaltig finanziell stimulieren? Ein Untersuchungsergebnis aus der Kieferorthopädie]. Gesundheitsokonomie und Qualitatsmanagement 2000;5(4):107‐11. [Google Scholar]
- Hennig CW, Crabtree CR, Baum D. Mental health CPR: peer contracting as a response to potential suicide in adolescents. Archives of Suicide Research 1998;4(2):169‐87. [Google Scholar]
- Jeffery RW, Gerber WM, Rosenthal BS, Lindquist RA. Monetary contracts in weight control: effectiveness of group and individual contracts of varying size. Journal of Consulting and Clinical Psychology 1983;51(2):242‐8. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Bjornson‐Benson WM, Rosenthal BS, Lindquist RA, Johnson SL. Behavioral treatment of obesity with monetary contracting: two‐year follow‐up. Addictive Behaviors 1984;9(3):311‐3. [DOI] [PubMed] [Google Scholar]
- Jeffrey DB, Christensen ER. Behavior therapy versus "will power" in the management of obesity. Journal of Psychology 1975;90(2 half):303‐11. [DOI] [PubMed] [Google Scholar]
- Johnson CC, Nicklas TA, Arbeit ML, Harsha DW, Mott DS, Hunter SM, et al. Cardiovascular intervention for high‐risk families: the Heart Smart Program. Southern Medical Journal 1991;84(11):1305‐12. [DOI] [PubMed] [Google Scholar]
- Jones RN, O'Brien P, McMahon WM. Contracting to lower precaution status for child psychiatric inpatients. Journal of Psychosocial Nursing & Mental Health Services 1993;31(1):6‐10, 30‐1. [DOI] [PubMed] [Google Scholar]
- Kim BE, Rhee HY. A study of the effects of health contracts on the performance level for activities of daily living in the hemiplegic patients. Kanho‐Hakhoe‐Chi 1991;21:63‐78. [DOI] [PubMed] [Google Scholar]
- Laidlaw JK, Beeken JE, Whitney FW, Reyes AA. Contracting with outpatient hemodialysis patients to improve adherence to treatment. ANNA Journal 1999;26(1):37‐40. [PubMed] [Google Scholar]
- Leslie M, Schuster PA. The effect of contingency contracting on adherence and knowledge of exercise regimens. Patient Education & Counseling 1991;18(3):231‐41. [Google Scholar]
- Lierman LM, Young HM, Powell‐Cope G, Georgiadou F, Benoliel JQ. Using social support to promote breast self‐examination performance. Oncology Nursing Forum 1994;21(6):1051‐7. [PubMed] [Google Scholar]
- Lowe JB, Windsor R, Balanda KP, Woodby L. Smoking relapse prevention methods for pregnant women: a formative evaluation. American Journal of Health Promotion 1997;11(4):244‐6. [DOI] [PubMed] [Google Scholar]
- Messina N, Farabee D, Rawson R. Treatment responsivity of cocaine‐dependent patients with antisocial personality disorder to cognitive‐behavioral and contingency management interventions. Journal of Consulting and Clinical Psychology 2003;1(2):320‐9. [DOI] [PubMed] [Google Scholar]
- Miller NH. Physical activity: one approach to the primary prevention of hypertension. AAOHN Journal 1995;43(6):319‐26. [PubMed] [Google Scholar]
- Napolitan SM. An evaluation of the effectiveness of early psychoeducational orientation and home visit intervention for first‐time caregivers of stroke patients [PhD thesis]. Chicago (IL): The University of Chicago, 1999. [Google Scholar]
- Neale AV. Behavioural contracting as a tool to help patients achieve better health. Family Practice 1991;8(4):336‐42. [DOI] [PubMed] [Google Scholar]
- Neuberger GB, Smith KV, Black SO, Hassanein R. Promoting self‐care in clients with arthritis. Arthritis Care & Research 1993;6(3):141‐8. [DOI] [PubMed] [Google Scholar]
- Norton RS, Powers RB. Commitment contingencies in the behavioral treatment of obesity. Annual Convention of the Rocky Mountain Psychological Association (50th, Tucson, AZ, April 9‐12, 1980). 1980. [Google Scholar]
- Ordman AM, Kirschenbaum DS. Cognitive‐behavioral therapy for bulimia: an initial outcome study. Journal of Consulting & Clinical Psychology 1985;53(3):305‐13. [DOI] [PubMed] [Google Scholar]
- Pantalon MV, Chawarski MC, Carroll KM, Schottenfeld RS. Therapeutic contracting for combined opioid and cocaine dependence: a pilot study. Drug and Alcohol Dependence 2001;63(Suppl 1):119. [Google Scholar]
- Paxton R. The effects of a deposit contract as a component in a behavioural programme for stopping smoking. Behaviour Research & Therapy 1980;18(1):45‐50. [DOI] [PubMed] [Google Scholar]
- Radojevic V, Nicassio PM, Weisman MH. Behavioral intervention with and without family support for rheumatoid arthritis. Behavior Therapy 1992;23(1):13‐30. [Google Scholar]
- Resnicow K, Royce J, Vaughan R, Orlandi MA, Smith M. Analysis of a multicomponent smoking cessation project: what worked and why. Preventive Medicine 1997;26(3):373‐81. [DOI] [PubMed] [Google Scholar]
- Sagawa M, Oka M, Chaboyer W. The utility of cognitive behavioural therapy on chronic haemodialysis patients' fluid intake: a preliminary examination. International Journal of Nursing Studies 2003;40(4):367‐73. [DOI] [PubMed] [Google Scholar]
- Sand P, Berni R. An incentive contract for nursing home aides. American Journal of Nursing 1974;74(3):475‐7. [PubMed] [Google Scholar]
- Saxon AJ, Calsyn DA, Kivlahan DR, Roszell DK. Outcome of contingency contracting for illicit drug use in a methadone maintenance program. Drug & Alcohol Dependence 1993;31(3):205‐14. [DOI] [PubMed] [Google Scholar]
- Schinke S, Pand R, Sheldon D. Interpersonal skill training in groups. Journal of Counseling Psychology 1976;23(5):442‐8. [Google Scholar]
- Solanto MV, Jacobson MS, Heller L, Golden NH, Hertz S. Rate of weight gain of inpatients with anorexia nervosa under two behavioral contracts. Pediatrics 1994;93(6 Pt 1):989‐91. [PubMed] [Google Scholar]
- Stuart RB, Jayaratne S, Tripodi T. Changing adolescent deviant behaviour through reprogramming the behaviour of parents and teachers: an experimental evaluation. Canadian Journal of Behavioural Science/Revue canadienne des sciences du comportement 1976;8(2):132‐44. [Google Scholar]
- Toseland RW, Kabat D, Kemp K. Evaluation of a smoking‐cessation group treatment program. Social Work Research & Abstracts 1983;19(1):12‐9. [Google Scholar]
- Tusel DJ, Piotrowski NA, Sees KL, Reilly PM, Banys P, Meek P, Hall SM. Contingency contracting for illicit drug use wit opioid addicts in methadone treatment. NIDA Research Monograph 1994;153:155. [Google Scholar]
- Ureda JR. The effect of contract witnessing on motivation and weight loss in a weight control program. Health Education Quarterly 1980;7(3):163‐85. [DOI] [PubMed] [Google Scholar]
- Dover LJ. Use of nurse‐client contracting to reduce risk of unintended pregnancy in an adolescent population. APHA annual conference 1985. 1985:15. [Google Scholar]
- Villano CL, Rosenblum A, Magura S, Fong C. Improving treatment engagement and outcomes for cocaine‐using methadone patients. American Journal of Drug & Alcohol Abuse 2002;28(2):213‐30. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Green L, Huxtable K. Blood glucose monitoring by diabetic adolescents: compliance and metabolic control. Health Psychology 1989;8(3):267‐84. [DOI] [PubMed] [Google Scholar]
- Zandee GL, Oermann MH. Effectiveness of contingency contracting: component of a worksite weight loss program. AAOHN Journal 1996;44(4):183‐8. [PubMed] [Google Scholar]
Additional references
- Anderson RJ, Reed G, Kilkr LM. Compliance in elderly hypertensives. Clinical Therapuetics 1982;5(Special issue):13‐24. [PubMed] [Google Scholar]
- Arnet I. Individualisation of compliance. Therapeutische Umschau 2000;57(9):552‐6. [DOI] [PubMed] [Google Scholar]
- Biller N, Caudill M. Commentary: contracts, opioids and the management of chronic nonmalignant pain. Journal of Pain and Symptom Management 1999;17(2):144‐5. [PubMed] [Google Scholar]
- Boehm S, Schlenk EA, Funnell MM, Powers H, Ronis Dl. Predictors of adherence to nutrition recommendations in people with non‐insulin‐dependent diabetes mellitus. Diabetes Educator 1997;23(2):157‐65. [DOI] [PubMed] [Google Scholar]
- Brus HLM, Laar MAFJ, Taal E, Rasker JJ, Wiegman O. Effects of patient education on compliance with basic treatment regimens and health in recent onset active rheumatoid arthritis. Annals of Rheumatic Diseases 1998;57:146‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean?. Social Sciences and Medicine 1997;44(5):681‐92. [DOI] [PubMed] [Google Scholar]
- Chowdhury AMR, Chowdhury S, Islam MN, Islam A, Vaughan JP. Control of tuberculosis by community health workers in Bangladesh. Lancet 1997;350:169‐72. [DOI] [PubMed] [Google Scholar]
- Chistiensen‐Szalanski JJJ, Northcraft GB. Patient compliance behavior: the effects of time on patients' values of treatment regimens. Social Science and Medicine 1985;21(3):263‐73. [DOI] [PubMed] [Google Scholar]
- Cleemput I, Kesteloota K, DeGeesta S. A review of the literature on the economics of noncompliance. Health Policy 2002;59(1):65‐94. [DOI] [PubMed] [Google Scholar]
- Cox K, Stevenson F, Britten N, Dundar Y. A systematic review of communication between patients and health care professionals about medicine‐taking and prescribing. GKT Concordance Unit. Guy's King's and St Thomas' School of Medicine (http://www.npc.co.uk/med_partnership/resource/major‐reviews/systematic‐review.html) May 2004 (accessed 7 February 2007). [DOI] [PMC free article] [PubMed]
- Cupples SA, Steslow B. Use of behavioral contingency contracting with heart transplant candidates. Progress in Transplantation 2001;11(2):137‐44. [DOI] [PubMed] [Google Scholar]
- Developing Patient Partnerships. Patients forced to wait because of missed GP appointments. DPP Media Release, http://www.dpp.org.uk/2003; Vol. August.
- Dunbar JM, Marshall GD, Hovell F. Behavioral strategies for improving compliance. In: Haynes TB, Taylor DW, Sackett DL editor(s). Compliance in health care. Baltimore: Johns Hopkins University Press, 1979:174‐90. [Google Scholar]
- Evangelista LS, Dracup K. A closer look at compliance research in heart failure patients in the last decade. Progress in Cardiovascular Nursing 2000;15(3):97‐103. [DOI] [PubMed] [Google Scholar]
- Fishman SM, Bandman TB, Edwards A, Borsook D. The Opioid Contract in the management of chronic pain. Journal of Pain and Symptom Management 1999;18(1):27‐37. [DOI] [PubMed] [Google Scholar]
- Fishman SM, Mahajan G, Jung SW, Wilsey BL. The trilateral opioid contract: bridging the pain clinic and the primary care and the primary care physician through the opioid contract. Journal of Pain and Symptom Management 2002;24(3):335‐44. [DOI] [PubMed] [Google Scholar]
- Fishman SM, Kreis PG.\. The opioid contracts. The Clinical Journal of Pain 2002;18(4):S70‐5. [DOI] [PubMed] [Google Scholar]
- Gordis L. Conceptual and methodologic problems in measuring patient compliance. In: Haynes TB, Taylor DW, Sackett DL editor(s). Compliance in health care. Baltimore: Johns Hopkins University Press, 1979:23‐45. [Google Scholar]
- Greenan‐Fowler E, Powell C, Varni JW. Behavioral treatment of adherence to therapeutic exercise by children with hemophilia. Archives of physical medicine and rehabilitation 1987;68(12):846‐9. [PubMed] [Google Scholar]
- Hayes RA, Efron LA, Richman GS, Harrison KA, Aguilera EL. The effects of behavioural contracting and preferred reinforcement on appointment keeping. Behaviour Change 2000;17(2):90‐6. [Google Scholar]
- Haynes RB. Introduction. In: Haynes TB, Taylor DW, Sackett DL editor(s). Compliance in health care. Baltimore: Johns Hopkins University Press, 1979:1‐7. [Google Scholar]
- Haynes RB. Determinants of compliance: the disease and the mechanics of treatment. In: Haynes TB, Taylor DW, Sackett DL editor(s). Compliance in health care. Baltimore: Johns Hopkins University Press, 1979:49‐62. [Google Scholar]
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000011.pub3] [DOI] [PubMed] [Google Scholar]
- Heinssen RK, Levendusky PG, Hunter R. Therapeutic contracting with the seriously mentally ill. American Psychologist 1995;50(7):522‐32. [DOI] [PubMed] [Google Scholar]
- Heneghan CJ, Glasziou P, Perera R. Reminder packaging for improving adherence to self‐administered long‐term medications. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005025.pub2] [DOI] [PubMed] [Google Scholar]
- Hey K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004307.pub2] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2006, issue 4.
- Hulka BS. Patient‐clinician interactions and compliance. In: Haynes TB, Taylor DW, Sackett DL editor(s). Compliance in health care. Baltimore: Johns Hopkins University Press, 1979:63‐77. [Google Scholar]
- Janz NK, Becker MH, Hartman PE. Contingency contracting to enhance patient compliance: a review. Patient Education and Counseling 1984;5(4):165‐78. [DOI] [PubMed] [Google Scholar]
- Jones G. Prescribing and taking medicines: concordance is a fine theory but is mostly not being practised. BMJ 2003;327:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MT, Han HR, Hill MN, Rose L, Roary M. Depression, substance use, adherence behaviours, and blood pressure in urban hypertensive black men. Annals of Behavioral Medicine 2003;26(1):24‐31. [DOI] [PubMed] [Google Scholar]
- Macharia WM, Leon G, Rowe BH, Stephenson B, Haynes B. An overview of interventions to improve compliance with appointment keeping for medical services. JAMA 1992;267(13):1813‐7. [PubMed] [Google Scholar]
- Maher D, Uplekar M, Blanc L, Raviglione M. Treatment of tuberculosis: concordance is a key step. BMJ 2003;327:823‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins RB, Evans D, Zimmerman B, Clark NM. Patient compliance: are we wasting our time and don't know it?. American Review of Respiratory Disease 1992;146:1376‐7. [DOI] [PubMed] [Google Scholar]
- Miller WR, Wilbourne P. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction 2002;97(3):265‐44. [DOI] [PubMed] [Google Scholar]
- Molteni AL, Garske JP. Effects of contracts on childhood memory recollection: a controlled clinical analogue. Journal of Clinical Psychology 1983;39(6):914‐9. [DOI] [PubMed] [Google Scholar]
- Oxford English Dictionary Online. http://dictionary.oed.com/entrance.dtl. Oxford University Press, 2003.
- Paxton R. Prolonging the effects of deposit contracts with smokers. Behaviour Research and Therapy 1983;21(4):425‐33. [DOI] [PubMed] [Google Scholar]
- Quill TE. Partnership in patient case: a contractual approach. Annals of Internal Medicine 1983;98:228‐34. [DOI] [PubMed] [Google Scholar]
- Reda S, Makhoul S. Prompts to encourage appointment attendance for people with serious mental illness. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002085] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnikow K, Royce J, Vaughan R, Orlandi MA, Smith M. Analysis of multicomponent smoking cessation project: what worked and why. Preventive Medicine 1997;26(3):373‐81. [DOI] [PubMed] [Google Scholar]
- Reunion de consenso: Estrategias para un control eficaz de la hipertension arterial en España Barcelona, Espana, 14 y 15 de octubre de 2005. Nature Clinical Practice. Cardiovascular Medicine2006; Vol. Suppl 2:S2‐S7.
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta‐analysis. Medical Care 1998;36(8):1138‐61. [DOI] [PubMed] [Google Scholar]
- Rueda S, Park‐Wyllie LY, Bayoumi AM, Tynan AM, Antoniou TA, Rourke SB, Glazier RH. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001442.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In: Haynes TB, Taylor DW, Sackett DL editor(s). Compliance in health care. Baltimore: Johns Hopkins University Press, 1979:11‐22. [Google Scholar]
- Sanz EJ. Concordance and children’s use of medicines. BMJ 2003;327:858‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
- Sherman JM, Baumstein S, Hendeles L. Intervention strategies for children poorly adherent with asthma medications: one center's experience. Clinical Pediatrics 1991;40(5):253‐8. [DOI] [PubMed] [Google Scholar]
- Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta‐analysis of the association between adherence to drug therapy and mortality. BMJ 2006;333(15):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson FA, Barry CA, Britten N, Barber N, Bradley CP. Doctor‐patient communication about drugs: the evidence for shared decision making. Social Science and Medicine 2000;50:829‐40. [DOI] [PubMed] [Google Scholar]
- Townsend A, Hunt K, Wyke S. Managing multiple morbidity in mid‐life:a qualitative study of attitudes to drug use. BMJ 2003;327(7419):837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmink J, Matchaba P, Garner P. Directly observed therapy and treatment adherence. Lancet 2000;355:1345‐50. [DOI] [PubMed] [Google Scholar]
- Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003343.pub3] [DOI] [PubMed] [Google Scholar]
- World Health Organization. Adherence to long term therapies: evidence for action. http://www.emro.who.int/ncd/Publications/adherence_report.pdf 2003 (accessed 7 February 2007). [: ISBN 92 4 154599 2]


