3.
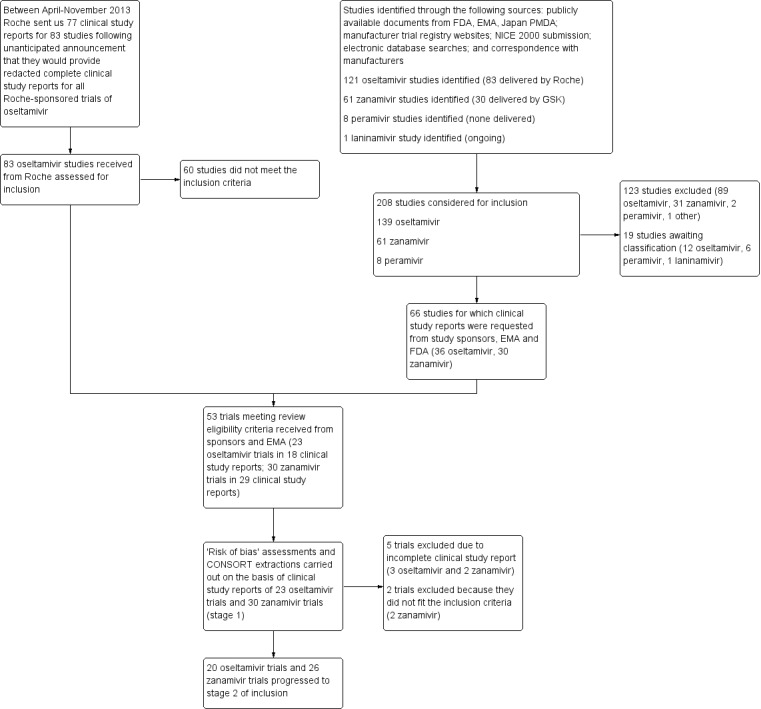
Flow diagram describing the number of studies identified, inclusion, exclusion and progression from identification to stage 1 to stage 2 of the review.
NB Because of the absence of trial programmes for both drugs listing all sponsored trials completed or underway, we had to rely on a variety of sources for the reconstruction of the trial programmes and retrieval of relevant clinical study reports. This complexity is reflected in the flowchart, illustrating the study selection process for this review. The two main pathways were the spontaneous release of 77 clinical full clinical study reports by Roche and the requests to regulatory authorities and GSK for all the relevant reports. There was overlap in trial reports retrieved following the different pathways
