Figure 3.
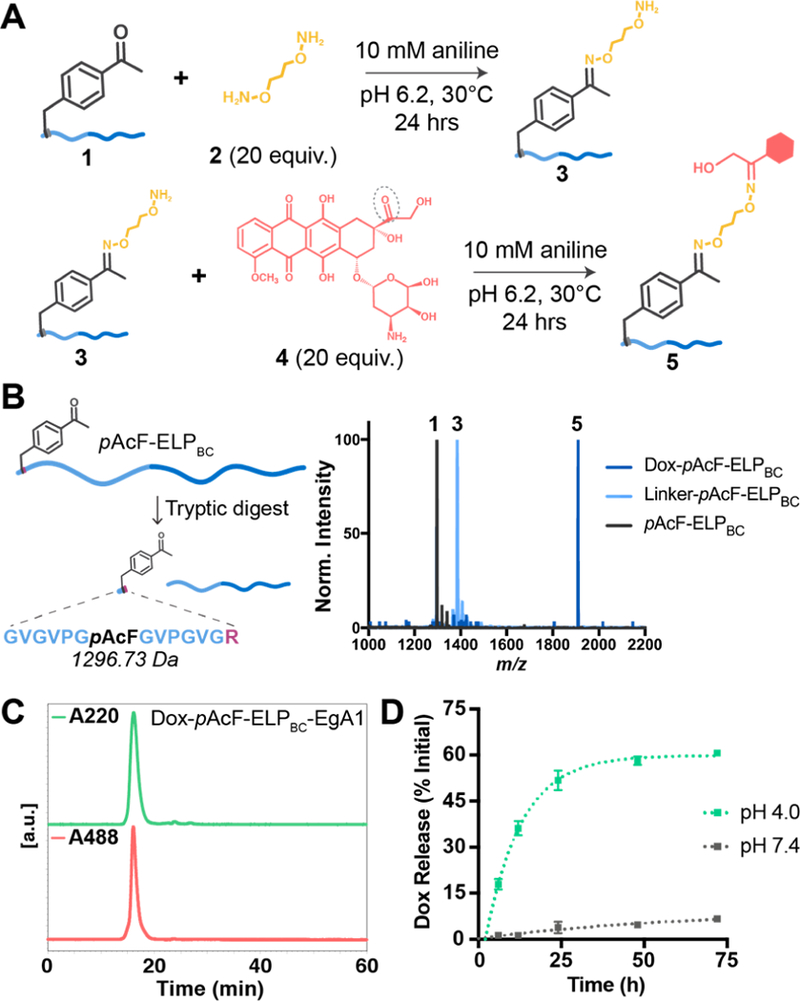
Confirming Dox attachment and acid-catalyzed release from pAcF-ELPBC and pAcF-ELPBC-EgA1. (A) Schematic of the two-step Dox conjugation to pAcF. First, pAcF-ELPBC 1 is reacted with the hydroxylamine linker 2 in the presence of 10 mM aniline catalyst to form the intermediate 3. This product is purified and reacted excess doxorubicin 4 under the same conditions to form the final conjugate 5. (B) The leader peptide containing pAcF can be liberated by tryptic digest from pAcF-ELPBC to confirm successful reaction steps via matrix assisted laser desorption ionization mass spectroscopy (MALDI-TOF): first, incorporation of pAcF in 1 (expected MW 1296.73 Da, observed 1296.65 Da), then modification with linker in 3 (expected MW 1384.84 Da, observed 1384.78 Da), and finally conjugation of Dox onto 5 (expected MW 1911.41 Da, observed 1910.15 Da). (C) Attachment of Dox to pAcF-ELPBC-EgA1 and purity of the final conjugate is confirmed using size exclusion chromatography (SEC), analyzing the spectrum at both A220 nm, to monitor the elution time of the conjugate, and A488 nm, the characteristic absorbance of Dox, to confirm attachment and purity. (D) pH-dependent release of Dox from Dox-pAcF-ELPBC-EgA1 is assessed by SEC at pH 4.0 (green) and pH 7.4 (gray).
