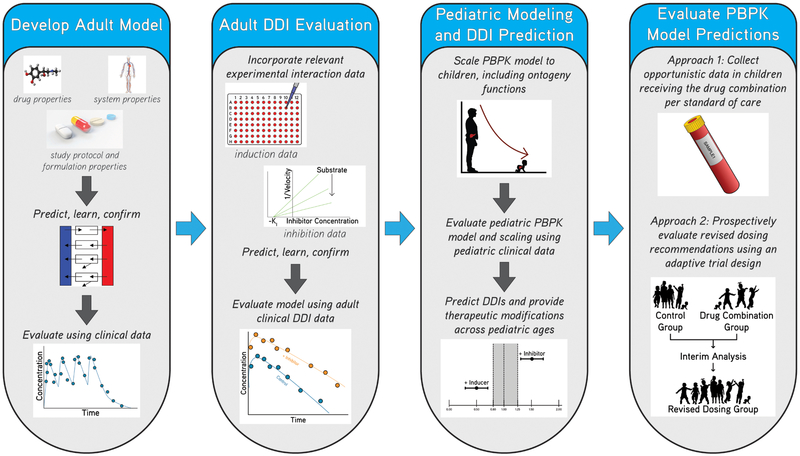
Figure 1: Application of physiologically-based pharmacokinetic (PBPK) modeling and simulation to predict drug-drug interaction (DDI) potential in pediatric patients.
Adult PBPK models can be developed incorporating drug-specific, system-specific, and study protocol and formulation properties, and then evaluated and further refined using adult clinical data. Next, DDI potential can be evaluated by incorporating in-vitro induction or inhibition parameters and then further refined using adult DDI data. Adult PBPK models can be scaled to pediatric patients including anthropomorphic and ontogeny functions, and then model performance and scaling can be evaluated using available pediatric data. Next, DDIs can be simulated in pediatric patients in order to provide therapeutic recommendations across pediatric ages likely to receive the drug. Finally, dosing recommendations can be evaluated using opportunistic pharmacokinetic data or using prospectively captured data from an adaptive trial. During adapative trials, efficacy and safety of the dosages and drug combinations can be monitored in pediatric patients throughout the trial at pre-specified times and the dosing regimen can be modified according to these interim study results.