Figure 2: Approaches to evaluate pediatric pharmacokinetic (PK) drug-drug interactions (DDIs) throughout pediatric drug development and post-marketing.
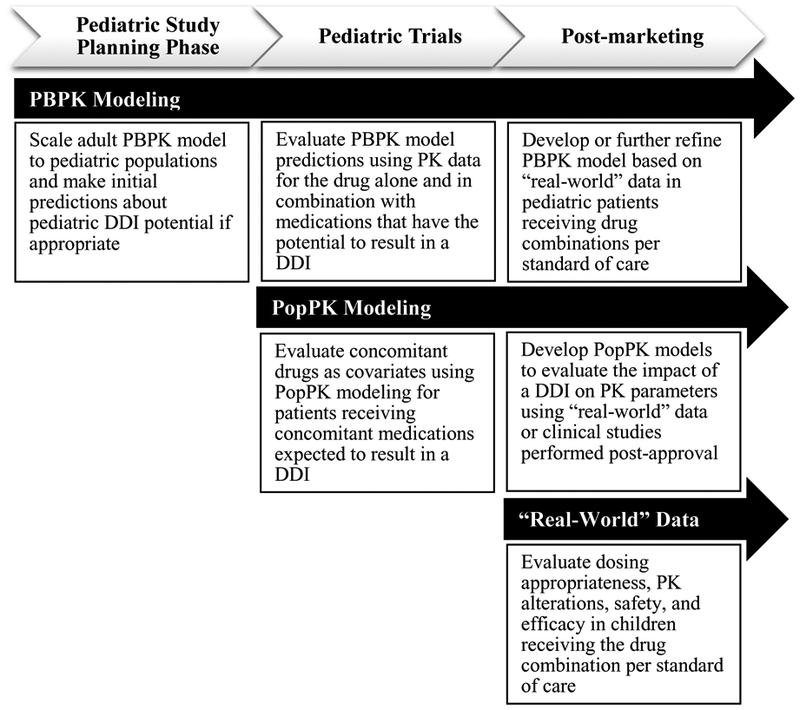
Prior to pediatric drug approval, physiologically-based pharmacokinetic (PBPK) modeling and simulation can investigate pediatric DDI potential and inform dose adjustments that can be evaluated through prospective pediatric trials, such as an adaptive trial. PBPK models can also be developed or further refined using “real-world” pediatric DDI data for marketed drugs. Once pediatric PK and coadministered drug data are available, PopPK models can be developed and concomitant drugs can be evaluated as predictors of inter-individual variability in PK parameters. Dosing simulations can then be performed based on the final population PK (PopPK) model to optimize dosing for children receiving the drug combination of interest. After drug approval, studies leveraging “real-world” data can be performed to evaluate dosing appropriateness, PK alterations, safety, and efficacy in children receiving the drug combination of interest per standard of care.
