Figure 3: A Speculative Model for the Epigenetic Behavior of Wild-type and Mutant Telosomes.
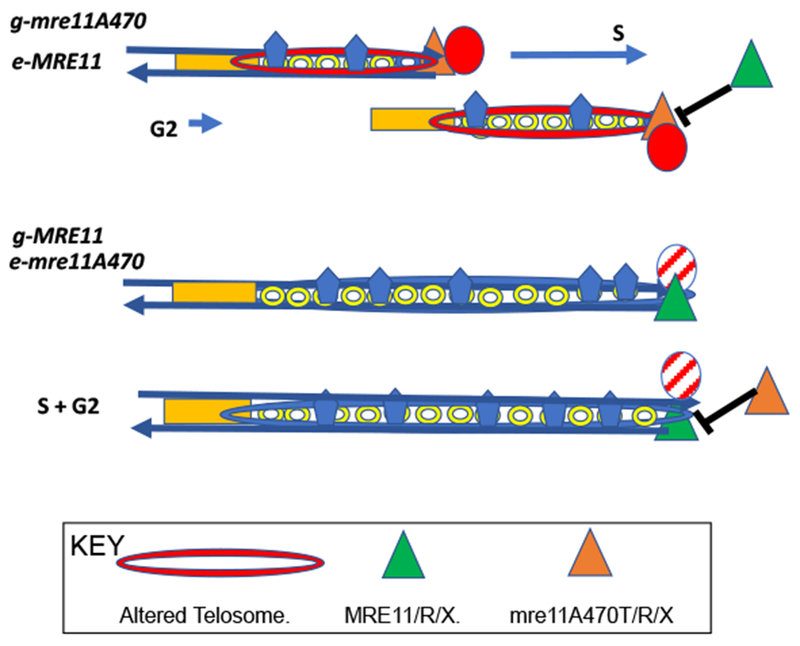
Phenotypes were determined after integration of the MRE11 allele as a single ectopic copy (e) in strains carrying an mre11A470 motif genomic allele (g) (g-mre11A470 e-MRE11). These cells led to mutant short telomeres. In contrast, the integration of mre11A470 motif allele (e) into MRE11 strains (g-MRE11 e-mre11A470) led to wild-type length telomeres. In the case of the specific mre11A470 allele tested, mre11A470T, telosome structure remained mutant in the g-mre11A470T e-MRE11 strains and g-MRE11 e-mre11A470T strains retained a wild-type telosome structure, despite the equal abundance of stable wild-type and mutant proteins in both cell types.
We propose a model based on a mutant telosome structure that has greater resistance to micrococcal nuclease (top, mutant; bottom, wild type), suggesting a more compact, tighter structure for mutant telosomes (red ovals). The central tenet of this model is the incompatibility of mutant Mre11A470T and wild-type Mre11 telosomes. Modified activities, epigenetic marking, or, a negative feedback loop, assist an S-phase microenvironment that favors formation of the mutant telomere. These epigenetic effects may play an evolutionary role in maintenance of the initial telosome after introduction of a modified form of the telosome.
