Table 1.
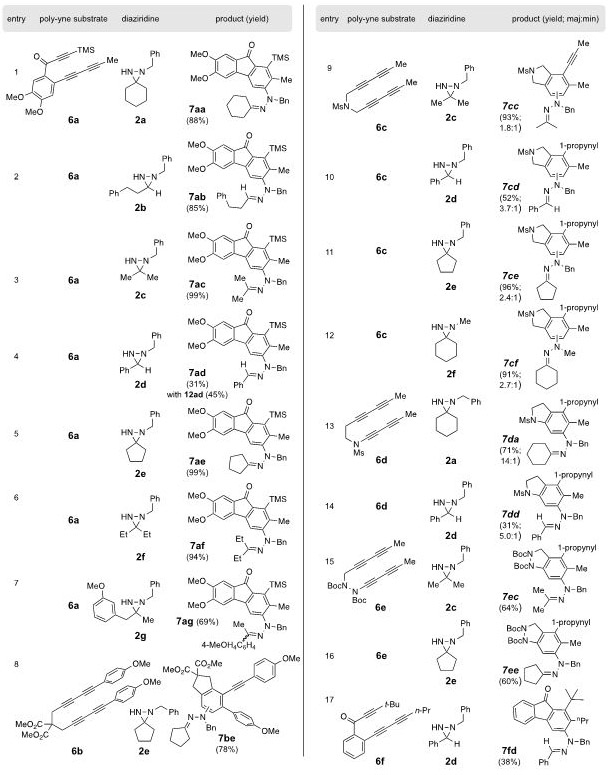 |
The structure number of each product contains two letters, the first indicating the poly-yne 6 and the second the diaziridine 2 of origin.
All reactions were performed in benzene having an initial [6] = 0.02 M and [2] = 0.04 M. Solutions were heated at 85–90 °C (external bath T) for 18-19 h, except for the case of the less reactive 6e, where the reaction time was 48 h.
