Figure 1.
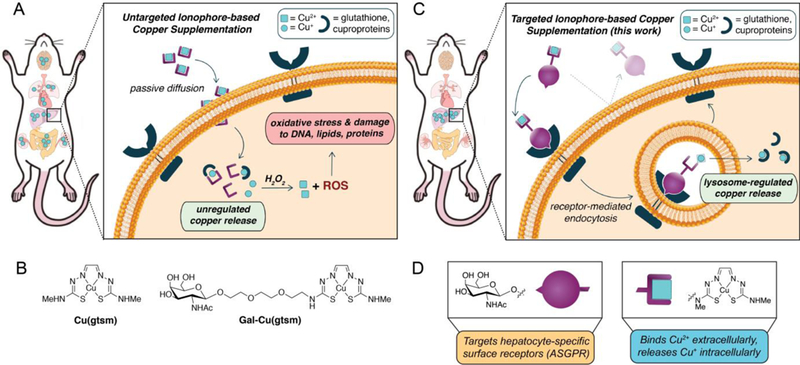
Schematic diagram comparing delivery methods for ionophore-based copper supplementation. (A) Conventional ionophores increase copper levels in many organs due to the non-specific nature of passive diffusion. For example, Cu(gtsm) releases copper intracellularly following reduction in the cytosolic medium. If too much copper is released such that it exceeds the cell’s copper buffering capacity, excess Cu+ can generate reactive oxygen species (ROS) through Fenton-like reactions to induce oxidative stress and damage to the cell. (B) Molecular structures for the untargeted Cu(gtsm) and targeted Gal-Cu(gtsm) ionophores studied here. (C) The targeted ionophore-based metal supplementation (TIMS) strategy presented here enables receptor-mediated metal accumulation with minimal off-target delivery, as shown by liver-selective copper supplementation. The hydrophilicity of the targeted ionophore precludes passive diffusion; ionophore internalization only occurs upon ligand-receptor recognition and endocytosis. Copper release is likely controlled by homeostatic cues at the level of the lysosome to enable regulated copper delivery. (D) The bifunctional ionophore design for Gal-Cu(gtsm) uses a triethyleneglycol linker to join the Cu(gtsm) moiety, which binds copper selectively and releases it upon intracellular reduction, with a GalNAc targeting group that is a specific ligand for ASGPR proteins expressed on the cell membranes of hepatocytes.
