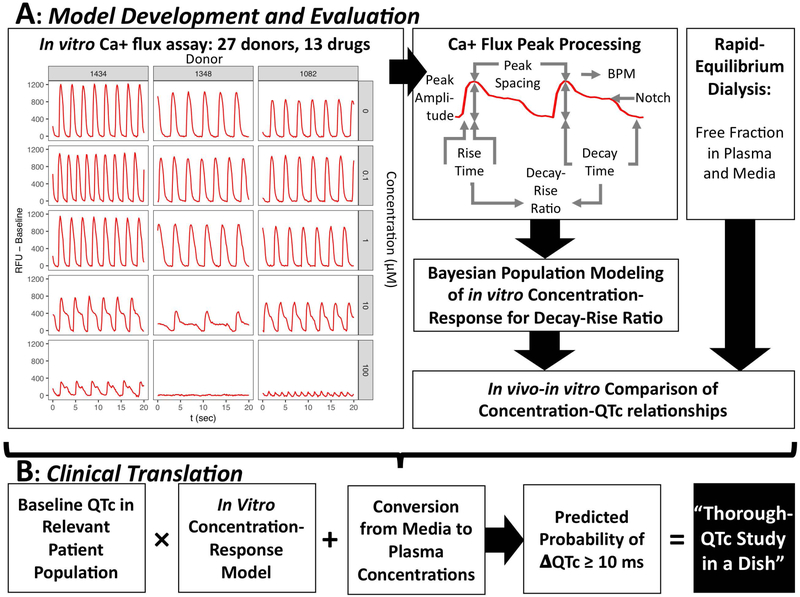
Figure 1. Overview of methods for predicting concentration-response for QTc prolongation and clinical translation to a “Through QTc Study in a Dish.”.
(A) Model development and evaluation. On the left are representative Ca2+ flux traces for cells derived from three donors at concentrations of 0, 0.1, 1, 10, and 100 uM disopyramide. Ca+ flux traces were processed to derive peak parameters, including the decay-rise ratio as the in vitro surrogate for the in vivo QTc. Concentration-response modeling using a Bayesian population approach was then conducted. The resulting concentration-response predictions were then compared to the in vivo concentration-response models, adjusting for differing free fractions in plasma and media. (B) Clinical translation to a Thorough QTc (TQT) Study. The in vitro concentration-response model expressing percent change as a function of media concentration is scaled first to ms change by using the baseline QTc in the relevant patient population, then by converting media concentrations to plasma concentrations. Then, using the posterior distributions of the Bayesian population model, the probability that the change in QTc (ΔQTc) is greater than or equal to 10 ms is calculated. This prediction is equivalent to the regulatory threshold determination in a traditional TQT study, which is 95% percent (one-sided) confidence that the change in QTc is no more than 10 ms.