Figure 8.
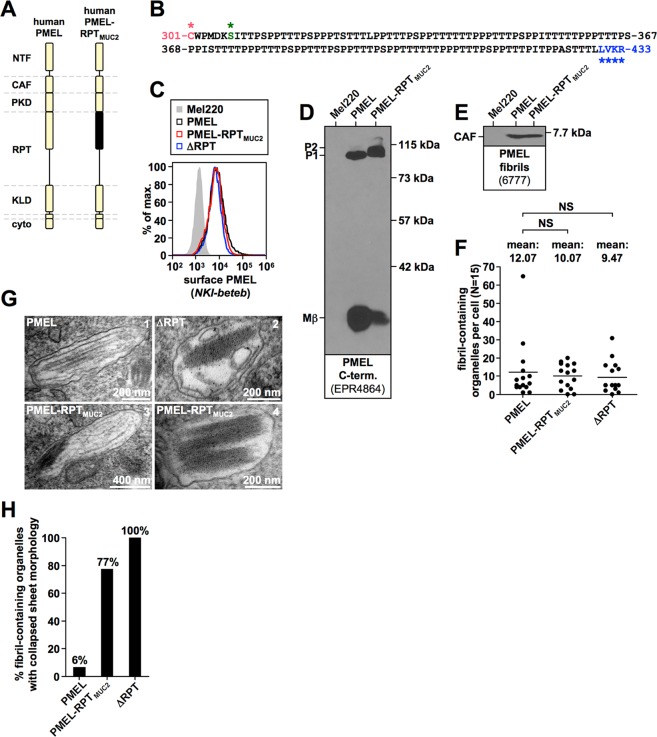
A randomly selected, O-glycosylated segment from MUC2 can partially substitute for the human RPT domain. (A) Schematic representation of the MUC2-chimeric PMEL construct PMEL-RPTMUC2. (B) Amino acid sequence of the MUC2 segment contained in PMEL-RPTMUC2. NetOGlyc 4.0 predicts 71 O-glycosylation sites within the MUC2 segment. (C) Flow cytometry analysis of the surface expression of human PMEL, human ΔRPT, and chimeric construct PMEL-RPTMUC2. (D,E) Western blot analysis of SDS-lysed total membranes using PMEL-specific antibodies EPR4864 (PMEL C-term.) (D) and 6777 (CAF) (E). (F,G) Quantitative EM analysis of Mel220 transfectants showing the number of fibril-containing organelles per cell [N = 15]. A One-Way ANOVA with Dunnett’s post test was used to determine whether means are statistically different from the wildtype human PMEL sample (NS, not significant). Representative electron micrographs are depicted (G). Note that some organelles in PMEL-RPTMUC2-expressing cells contain well-separated sheets (panel 3), which are never observed in ΔRPT-expressing cells (Supplementary Fig. S4H). (H) Quantification of the number of fibril containing organelles displaying the collapsed amyloid sheet phenotype.