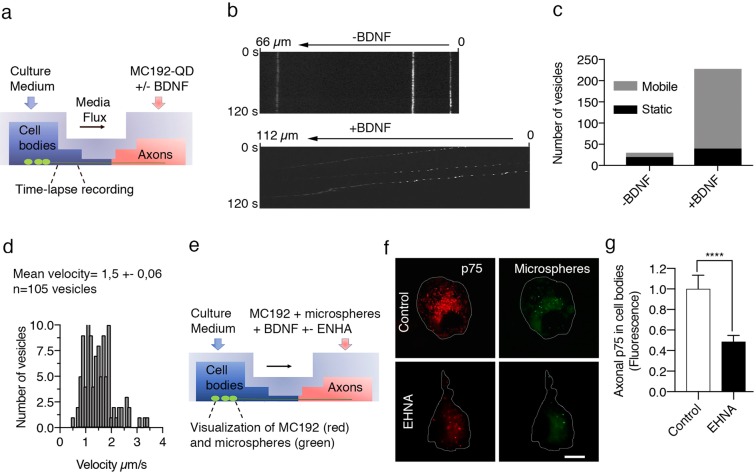
Figure 1.
Axonal p75 is retrogradely transported in a ligand- and dynein-dependent manner. (a) Illustration of the design of the experiment used to study retrograde transport and signaling in sympathetic neurons. (b) Kymographs of axonal p75 movement in response to BDNF. Compartmentalized sympathetic neuron cultures were treated with BDNF (200 ng/mL) and MC192-QD, which labeled the extracellular domain of p75, for 4 hours at 37 °C in the axon compartment before imaging. Upper panels show kymographs of retrograde p75 movement in the absence (−) and presence (+) of BDNF in the axon compartment. (c) The graph indicates the proportion of vesicles without (black) or with (gray) movement when axon chambers were treated without (−) or with (+) BDNF. Data were obtained from videos captured in three independent experiments. (d) Histogram showing the distribution of velocities measured from 105 moving vesicles. The mean velocity of p75 vesicles undergoing retrograde transport in the presence of BDNF was approximately 1.5 µm/second. (e) Illustration of the design of the experiment used to study the retrograde transport of p75 and its dependence on dynein. (f) Visualization of axonally labeled p75 in the cell bodies of compartmentalized sympathetic neurons. The axons of compartmentalized neuronal cultures were treated with BDNF and MC192-Alexa Fluor 594 (to label p75, red) and microspheres labeled with Alexa Fluor 488 (to label compartmentalized neurons, green) for 16 hours at 37 °C in the absence (control) or presence of the dynein inhibitor EHNA (erythro-9-(2-hydroxy-3-nonyl). Scale bar, 10 μm. (g) Levels of axonally labeled p75 in the absence (control) or presence of EHNA. Data from 120 cells in four different compartmentalized chambers were quantified. Microspheres labeled with Alexa Fluor 488 were added 24 hours before the BDNF treatment and maintained in the culture throughout the experiment. Statistically significant differences were analyzed using a two-tailed Mann-Whitney test. ****p < 0,0001.