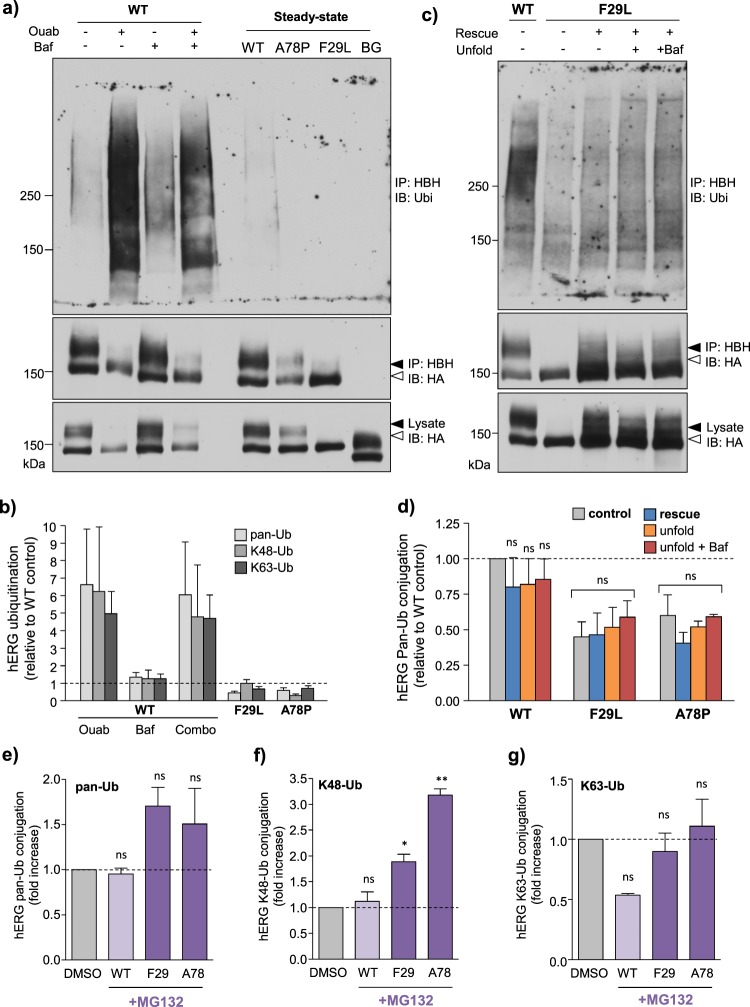
Figure 7.
PAS-mutant hERG is negligibly ubiquitinated at post-Golgi compartments. (a) PAS mutants are not significantly ubiquitinated under steady-state conditions. hERG-HBH were affinity-isolated on monomeric avidin beads. Ubiquitination detected by immunoblotting with a pan-Ub Ab recognizing mono- and poly-Ub linked chains (P4D1). Destabilization of WT-hERG by acute intracellular K+-depletion with ouabain (ouab, 3 h at 300 nM) elicited profound ubiquitination (left). In contrast, PAS-mutant channels did not exhibit a similar increase in ubiquitination (right). Similar results obtained when probed with K48-Ub and K63-Ub chain specific Abs (Supplementary Fig. S10). Non-specific binding assessed in HeLa cells expressing non-HBH-tagged WT-hERG (rightmost lane). Baf: Bafilomycin A1 (200 nM). (b) Cells treated as in (a) and ubiquitination detected by ELISA. HBH-tagged hERG immobilized onto streptavidin plates and denatured in 8 M urea. Total ubiquitination (mono and polyUb) and K48/K63-Ub linked chains were detected by ELISA, normalized for hERG (HA) signal and expressed as a fraction relative to untreated WT-hERG control. (c,d) Attenuated PAS-mutant hERG ubiquitination is not dependent on mature protein expression. Cells expressing hERG-HBH were subject to low-temperature rescue (26 °C for 24 h) and subsequent unfolding (37 °C for 3 h) in the presence/absence of Bafilomycin A1 (Baf, 200 nM). hERG was affinity-isolated and total ubiquitination detected by immunoblotting (c) or ELISA (d) using a pan-Ub Ab (P4D1). Similar results obtained when probed with K48-Ub and K63-Ub chain specific Abs (Supplementary Fig. S10). (e–g) Proteasomal inhibition causes accumulation of K48-polyubiquitinated PAS-mutant hERG. hERG ubiquitination evaluated by ELISA following acute proteasomal inhibition with MG132 (10 µM for 3 h). Ubiquitination expressed as fold increase over untreated (DMSO) control. Proteasomal inhibition tended to increase total ubiquitination (e) and significantly increased K48-polyubiquitination of mutant hERGs (f). K63-polyubiquitination was unaffected (g). *P < 0.05, **P < 0.01, ***P < 0.001, n.s. = no significant difference (See Methods for explanation of statistical analysis). Full-length anti-Ub immunoblots shown here. Uncropped αHA blots in Supplementary Fig. S12. Solid line: different parts of the same gel. White space: separate gels.