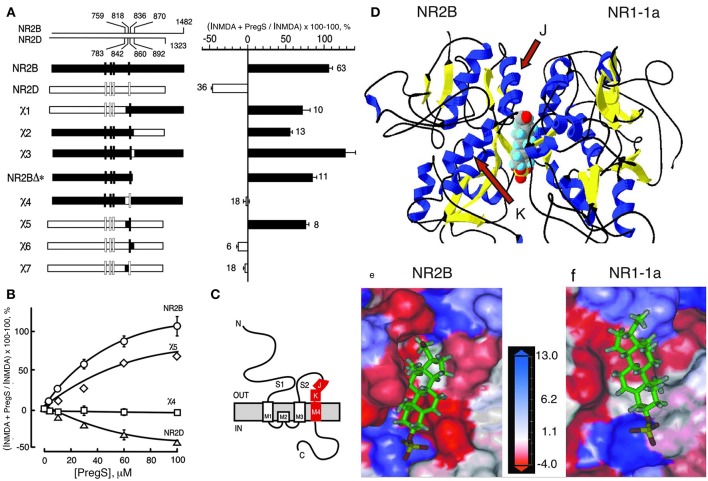
Figure 3.
Steroid modulatory domain of NMDARs. (A) (Left) Schematic representation of wildtype NR2B, NR2D, and the NR2B, NR2D chimeras. The contribution of NR2B and NR2D to chimeras is depicted in black and white, respectively. The scales at the top indicate the residue numbers in the wildtype subunits at junctions. Vertical bars represent the four hydrophobic membrane domains. (Right) Percent increase in the NMDA glycine response (elicited by 300 μM NMDA and 50 μM glycine in oocytes expressing NR1-1a and NR2 subunits) in the presence of 100 μM PS is indicated. Error bars are SEMs. Numbers adjacent to the error bars indicate the number of oocytes used in the study. (B) Concentration–response curves of PregS modulation for receptors containing NR2B (○), NR2D (Δ), χ4 (), and χ5 (♢), were determined in the presence of saturating concentrations of NMDA (300 μM) and glycine (50 μM). The EC50 (NMDA) for NR1-1a χ4 and NR1-1a_NR2B are both 22 ± 1 μM, and EC50 (glycine) is 0.30 ± 0.02 and 0.10 ± 0.02 μM, respectively. (C) The topological representation of the NR2B subunit and the location of the identified segment are depicted in red. Membrane domains are denoted as M1–M4. The amino terminus (N) is located on the extracellular side and the carboxyl terminus (C) on the intracellular side of the plasma membrane. (D) Molecular modeling of potential binding pocket for PregS. The dimer comprising the S1/S2 domains of NR2B and NR1-1a is depicted in a 3D ribbon structure with helices colored in blue and sheets colored in yellow with PregS docked at the interface between the two subunits. Our finding that both J and K helices (see arrows) and M4 of the NR2B subunit are required to confer PS potentiation indicates that M4 is also critical in coupling allosteric modulation from extracellular binding regions to the gating mechanism. (E) Detailed view of the potential binding pocket for PregS on NR2B. (F) Detailed view of the potential binding pocket for PregS on NR1-1a. NR1-1a or NR2B have been removed from the models to show the hydrophobic pocket on NR2B (E) or NR1-1a (F), respectively. The receptor surface is colored according to a hydrophobicity scale with hydrophobic residues in red and charged residues in blue. PregS is depicted in a stick configuration and colored by the atom type with hydrogen in white, carbon in green, oxygen in red, and sulfur in yellow. Our finding that both J and K helices (see arrows) and M4 of the NR2B subunit are required to confer PS potentiation indicates that M4 is also critical in coupling allosteric modulation from extracellular binding regions to the gating mechanism [From Jang et al. (43) with Permission].