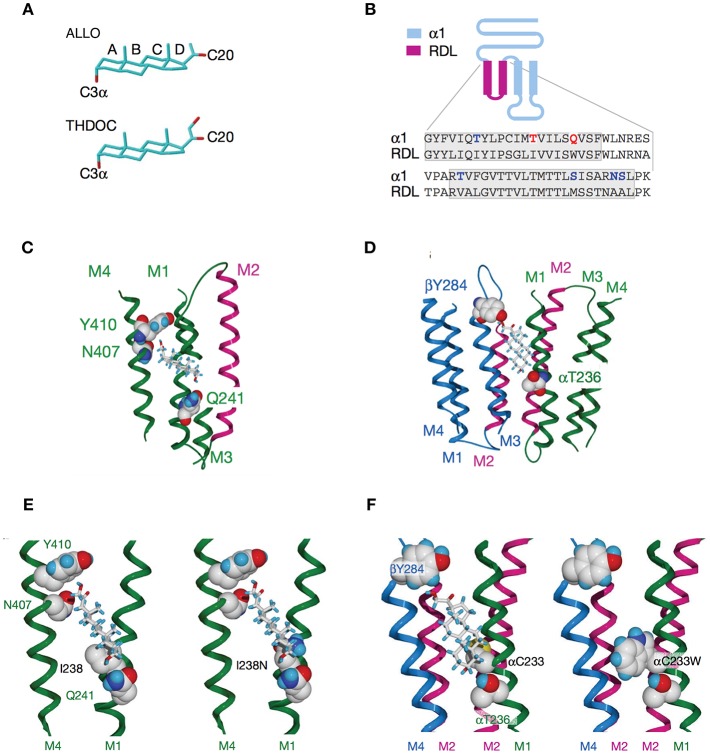
Figure 4.
Activation of GABAARs by neurosteroids, such as ALLO and THDOC depends on occupancy of both the activation and potentiation sites on the transmembrane domain. (A) Regulation of GABAAR, is dependent on the C3a hydroxyl group on the A-ring and the C20 ketone on the D-ring shown here in structures of ALLO and THDOC. (B) Neurosteroid activity is determined by α-subunit M1 domain residues. Replacement of membrane domains M1 through to the end of M2 in the murine α1 and β2 subunits with the corresponding sequence from the RDL subunit, forming the chimeras αR and βR, respectively, was the first modification used to established their GABAAR pharmacology. Potentiation and direct activation of GABAARs by THDOC and ALLO was abolished in chimeric receptors incorporating αR; receptors containing βR were indistinguishable from the wild type. Polar residues in α1 (blue) are in bold, with Thr 236 and Gln 241 (red) highlighted. The transmembrane domains are boxed. (C) Neurosteroid potentiation requires α-subunit M1 and M4 membrane domains. Ribbon structure of α subunit viewed from the lipid bilayer showing αGln 241, αAsn 407, and αTyr 410 docking with a THDOC molecule. The channel lining the M2 membrane domain is shown in purple (a section of M3 domain is omitted for clarity). (D) Neurosteroid activation binding site spans the β/α-subunit interface. View of transmembrane region (extracellular and cytoplasmic domains removed) with a bound THDOC molecule. Replacing αThr 236 with non-hydrogen-bonding isoleucine or valine reduces the agonist potency of ALLO and THDOC. (E) Neurosteroid potentiation requires α-subunit M1 and M4 membrane domains. Homology model of THDOC bound to the potentiation site between M1 and M4 membrane domains of the [David: should the following show alpha or beta symbol?] α subunit (M3 membrane domain removed from figure for clarity). Ile 238 is predicted to lie close to the A-ring of THDOC (left). Introduction of a similar-sized but polar side chain at residue 238, such as replacement with asparagine repels the steroid (right). (F) Replacement of Cys 233 (left) with tryptophan (right) increases the steric hindrance for THDOC binding to βTyr 284 and αThr 236 [From Figures 1, 3, 4 of Hosie et al. (109)].