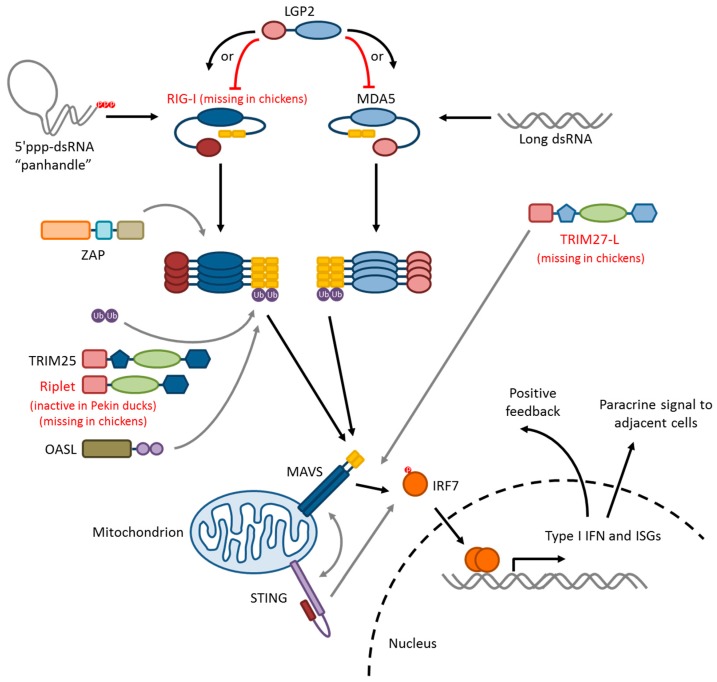
Figure 2.
Duck RIG-I-like receptor (RLR) family signaling pathway. The duck RLR family consists of three cytoplasmic receptors: RIG-I, MDA5, and LGP2. RIG-I and MDA5 detect viral RNA species and initiate interferon signaling. LGP2 does not possess a signaling domain and may act as a positive or negative regulator of the other two. RIG-I recognizes 5′-triphosphorylated RNA “panhandle” structures formed by the complementary ends of each influenza genome segment. MDA5 recognizes long double-stranded RNA. Ligand recognition leads to oligomerization of the receptors and releases the N-terminal caspase activation and recruitment domains (CARD) domains (shown as yellow rectangles) from repression by the C-terminal regulatory domain. Cytoplasmic zinc-finger antiviral protein (ZAP) protein may interact directly with RIG-I to stabilize RNA-bound tetramers. Short K-63-linked polyubiquitin chains synthesized by tripartite motif-containing protein 25 (TRIM25) stabilize the RIG-I CARD domain oligomers, which interact with similar CARD domains on mitochondrial MAVS. Duck Riplet appears to be missing its catalytic domain and likely cannot synthesize polyubiquitin chains. 2′-5′-oligoadenylate synthase-like protein (OASL) may stabilize RIG-I CARD domain oligomers with its own C-terminal ubiquitin-like domains in the absence of TRIM25-mediated ubiquitination. Activation of MAVS proteins leads them to multimerize in turn, and to serve as an assembly platform for a kinase cascade, which terminates in the phosphorylation of the transcription factor IRF7. A unique duck TRIM gene, TRIM27-L, promotes RIG-I signaling through an undefined interaction downstream of MAVS. Phosphorylated IRF7 dimerizes and is translocated to the nucleus where it initiates transcription of type I and type III interferon and interferon-stimulated genes. The expressed interferon is secreted to induce an antiviral state in the neighbouring cells and can also amplify RLR signaling in a positive feedback loop, since the pathway components are themselves interferon-inducible. RIG-I signaling activates stimulator of interferon gene (STING), an adaptor protein involved in sensing DNA viruses, which cross-talks with the RIG-I pathway. STING activation, in turn, prolongs the type I IFN response.