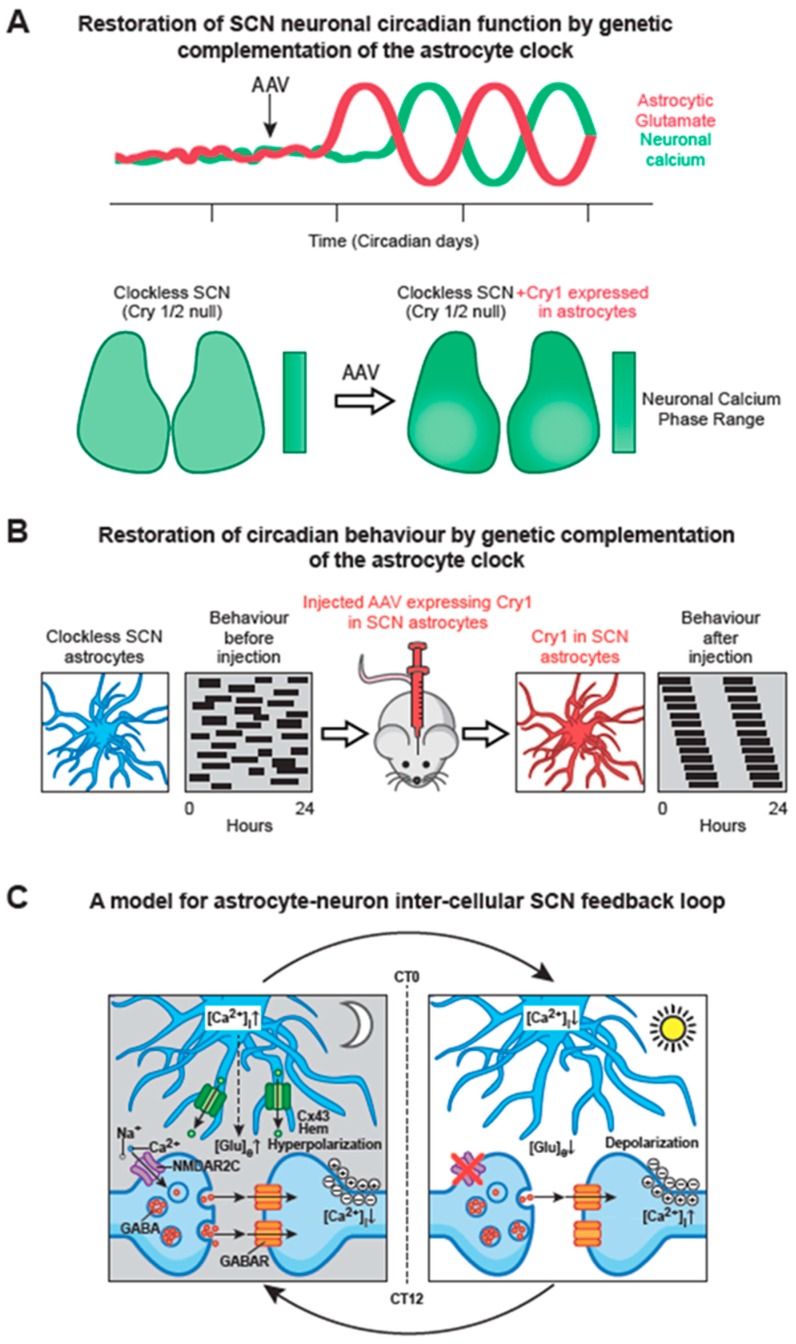
Figure 4.
Astrocytic-neuronal interplay in the generation of SCN circadian rhythms: a synthetic model. (A) Schematic illustration that AAV-mediated, selective expression of Cry1 in astrocytes of arrhythmic Cry1/2-null SCN is sufficient to initiate circadian rhythms of neuronal [Ca2+]i levels (green) notwithstanding the absence of a neuronal TTFL in these SCN. In parallel the newly circadian-competent astrocytes also initiate a circadian rhythm of [Glu]e (red), phased appropriately to peak in circadian night. Real-time imaging of slices further reveals that the astrocytically initiated calcium rhythm in neurons expresses the appropriate emergent spatio-temporal wave across the SCN circuit. (B) Selective expression of Cry1 in astrocytes of the SCN, achieved by stereotaxic injection of AAV vectors in arrhythmic Cry1/2-null adult mice, is sufficient to initiate and support circadian oscillations of locomotor behaviour. This likely reflects initiation of the SCN neuronal rhythms via restored rhythmic glutamate signalling from astrocytes, and subsequent engagement of downstream motor centres by the driven neurons. The long period of the restored rhythm is reflective of the cell-autonomous rhythm of Cry1-activated SCN astrocytes. (C) A model of astrocytic–neuronal interaction. During circadian night (left), extracellular glutamate concentrations ([Glu]e) are high, driven by astrocytic release of glutamate via Cx43 hemichannels and glutamate transporters (not shown). This activates presynaptic NR2C subunit-containing NMDA-type glutamate receptors (NMDAR2C), increasing the presynaptic intracellular Ca2+ concentration ([Ca2+]i) to facilitate GABA release, and thereby suppressing network-wide electrical activity of postsynaptic neurons. During circadian daytime (right), clearance of extracellular glutamate by reduced astrocytic glutamate release relieves GABAergic tone across the network, leading to depolarization and increased electrical firing of SCN neurons. Redrawn from [73].