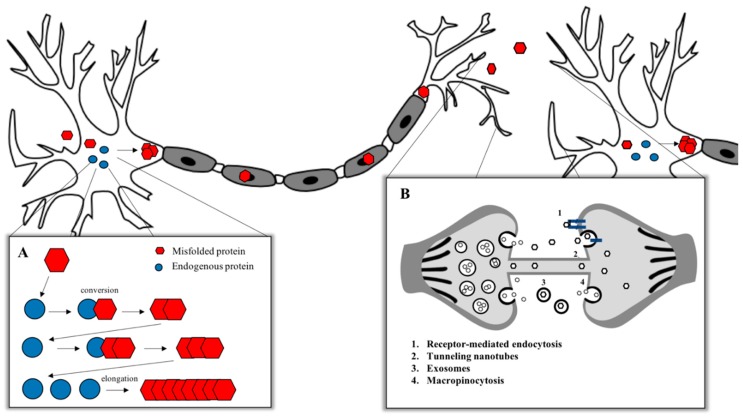
Figure 1.
A schematic illustration of the prion-like characteristics of aggregated proteins: (A) Once they have entered healthy neurons, amyloids (red hexagons) interact with their native counterparts (blue circles) and act as a template to seed their misfolding. The process is self-amplifying and leads to the formation of long fibrillar aggregates which are transported along the axons toward synaptic terminals. (B) The aggregates spread to neighbouring cells via multiple mechanisms, many of which require an active regulation by the cell itself. (1) Endocytosis: Prion-like proteins (synuclein) exploit membrane receptors (LAG3 [32] and prion protein [33]) or clathrin-dependent endocytosis (tau) to enter the cell cytoplasm. (2) Tunneling nanotubes (TNTs): TNTs are involved in the spreading of tau, prions, synuclein and Aβ [34]. (3) Exosomes: Exosomes have been found to be implicated in the spreading of pathological protein aggregates [35]. (4) Macropinocytosis: The tau protein is transferred through macropinocytosis mediated by heparin sulfate proteoglycans [36].